Question
Question: The compound B is 
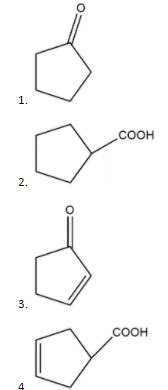
Solution
The compound given in the reaction is generally an ester along with the ketonic group. Esters are generally a chemical compound which is derived from an organic or inorganic acid in which hydroxyl group represented by OH is replaced by an alkyl i.e. (-O-) group.
Complete Step by step solution: Esters are generally defined as the group of chemical compounds which are formed by bonding of an alcohol group with a group of organic acids during this process it loses water molecules. Esters are also usually derived from carboxylic acids and can also be obtained by reaction of acid anhydride or acid halides with alcohols or by the reaction of salts of carboxylic acids with alkyl halides.
During this process in first step ester is converted into carboxylic acid and shown as:
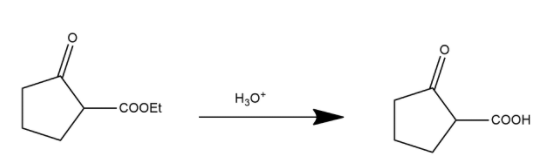
Here the ester group is converted into an acidic group and after this process the decarboxylation process occurs. Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide. It generally occurs in a reaction of carboxylic acids by removing a carbon atom from the carbon chain. The reverse process of decarboxylation is called carboxylation which is the addition of carbon dioxide.
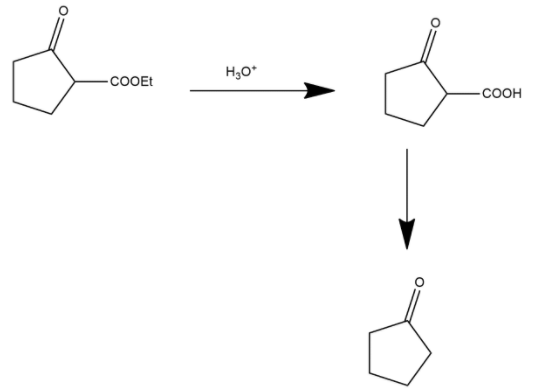
Hence we can say that option A is the correct answer.
Note: Generally enzymes which catalyze decarboxylation are called decarboxylases; it generally removes a carboxyl group from organic compounds. These further catalyze into amino acids, alpha keto acids and beta keto acids. Example of this is pyruvate.
