Question
Question: The compound B is: 
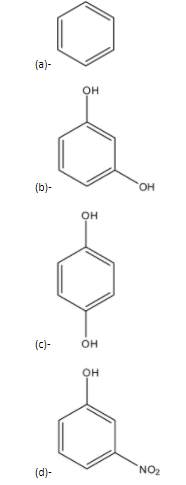
Solution
The reactant compound is m-dinitrobenzene or 1, 3-dinitrobenzene, and the reaction of m-dinitrobenzene with ammonium sulfide is an example of selective reduction and this reduction is popularly known as Zinin reduction. NaNO2+HCl is used to convert the amine group on the benzene group to the diazonium salt.
Complete step by step answer:
In the given reaction, the reactant compound is m-dinitrobenzene or we can say 1, 3-Dinitrobenzene. When two or more nitro groups are attached to the benzene ring there is a property of selective reduction, in which it is possible to reduce one of them without affecting the others and this is known as selective reduction. So when m-dinitrobenzene or 1, 3-dinitrobenzene reacts with sodium or ammonium sulfide i.e., (NH4)2S to forms m-nitroaniline. So the reaction is given below:
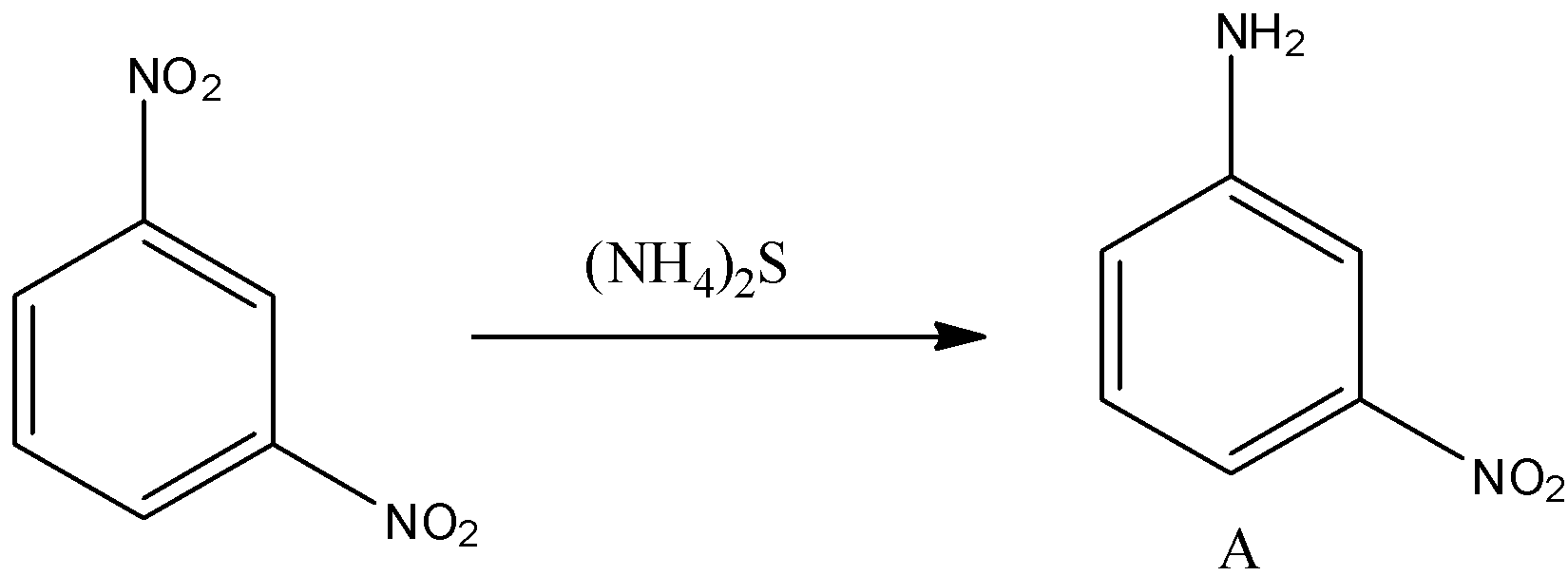
So the compound A is m-nitroaniline. Hence this reduction of nitro compounds with sulfide or polysulfide is known as Zinin reduction. Now this m-nitroaniline is reacted with nitrous acid i.e., the mixture of NaNO2 and HCl, and water (H2O). So when an amine group present on the benzene ring is treated with nitrous acid i.e., the mixture of NaNO2 and HCl, it will convert into diazonium salt. And this diazonium salt is treated with water, then the hydroxyl group will be substituted with the diazonium group in the benzene ring. So the reaction is given below:
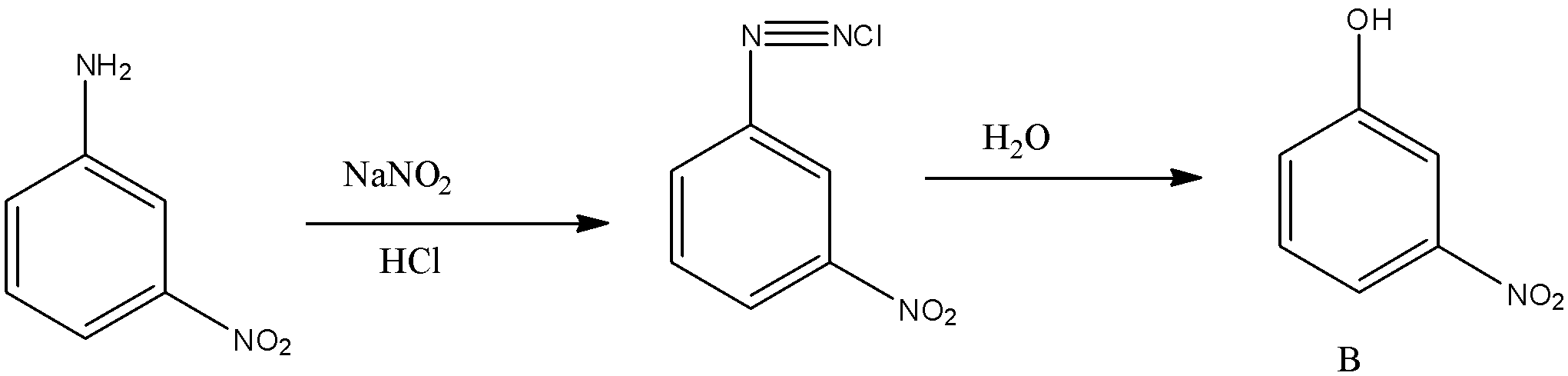
So the compound B will be m-nitrophenol.
Therefore the correct answer is an option (d).
Note: There are specific reagents used for selective reduction, if you use any other reducing agent they both the nitro group will get reduced to the amine group. The reaction of converting aniline to diazonium salts is known as diazotization.
