Question
Question: The compound A is: 
A. 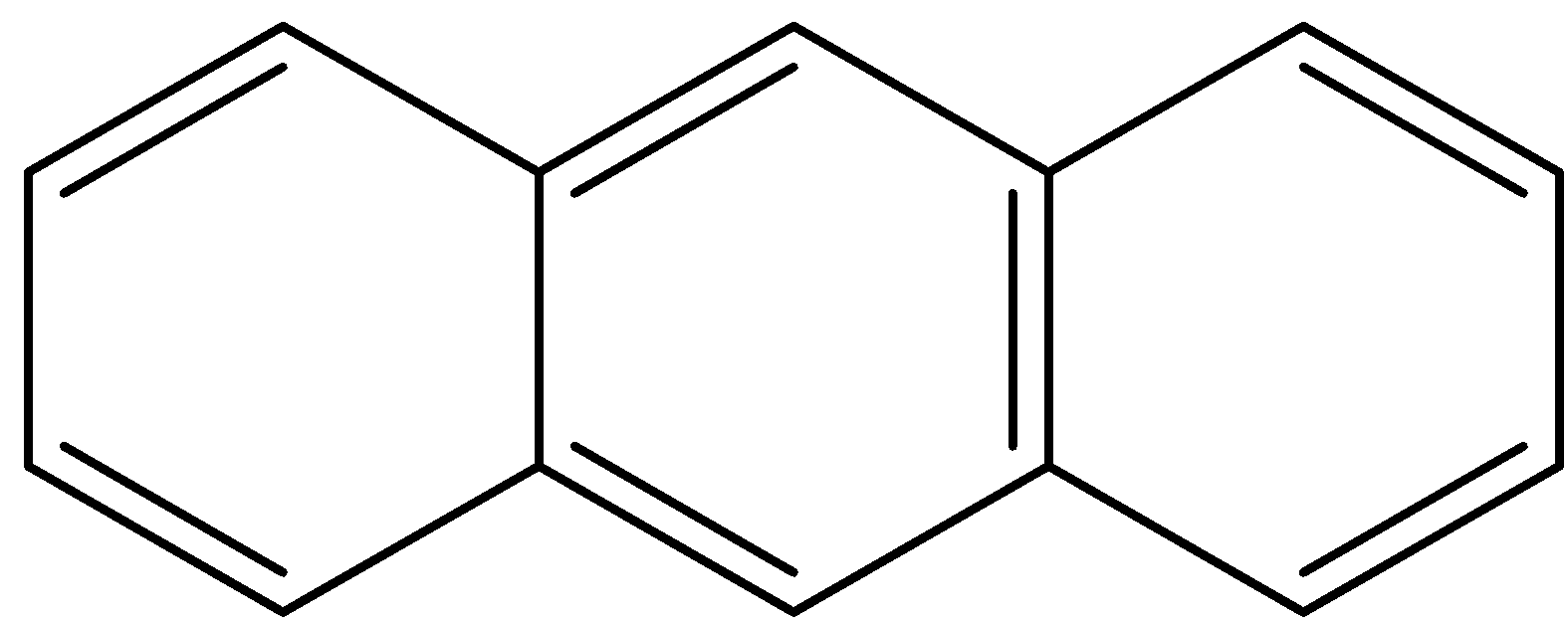
B. 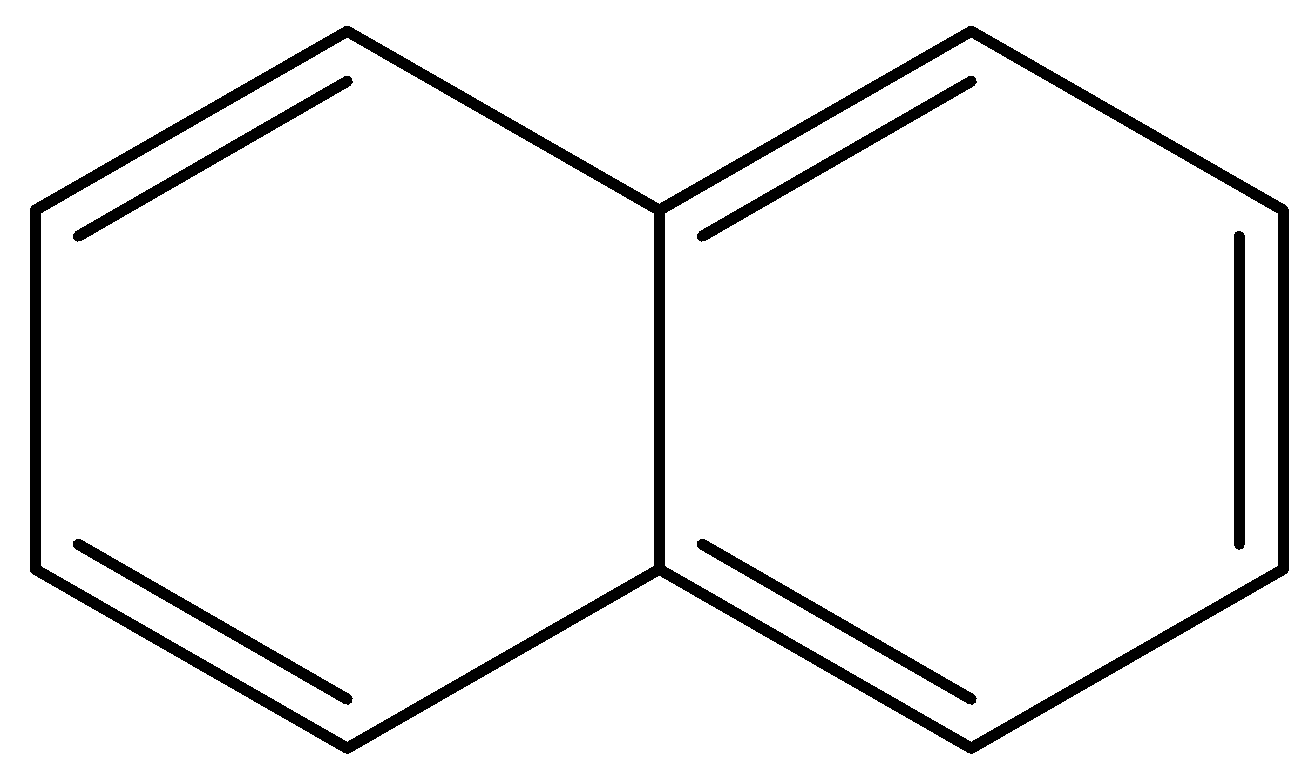
C. 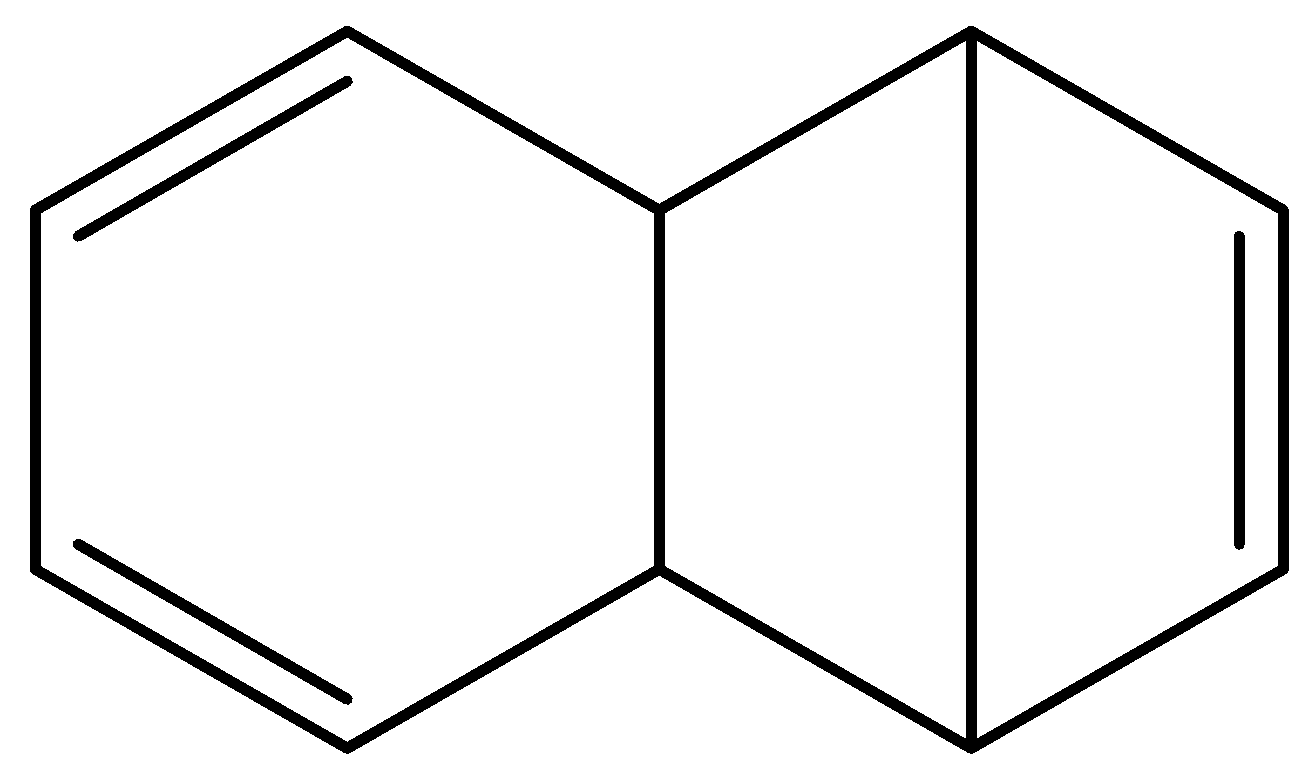
D. 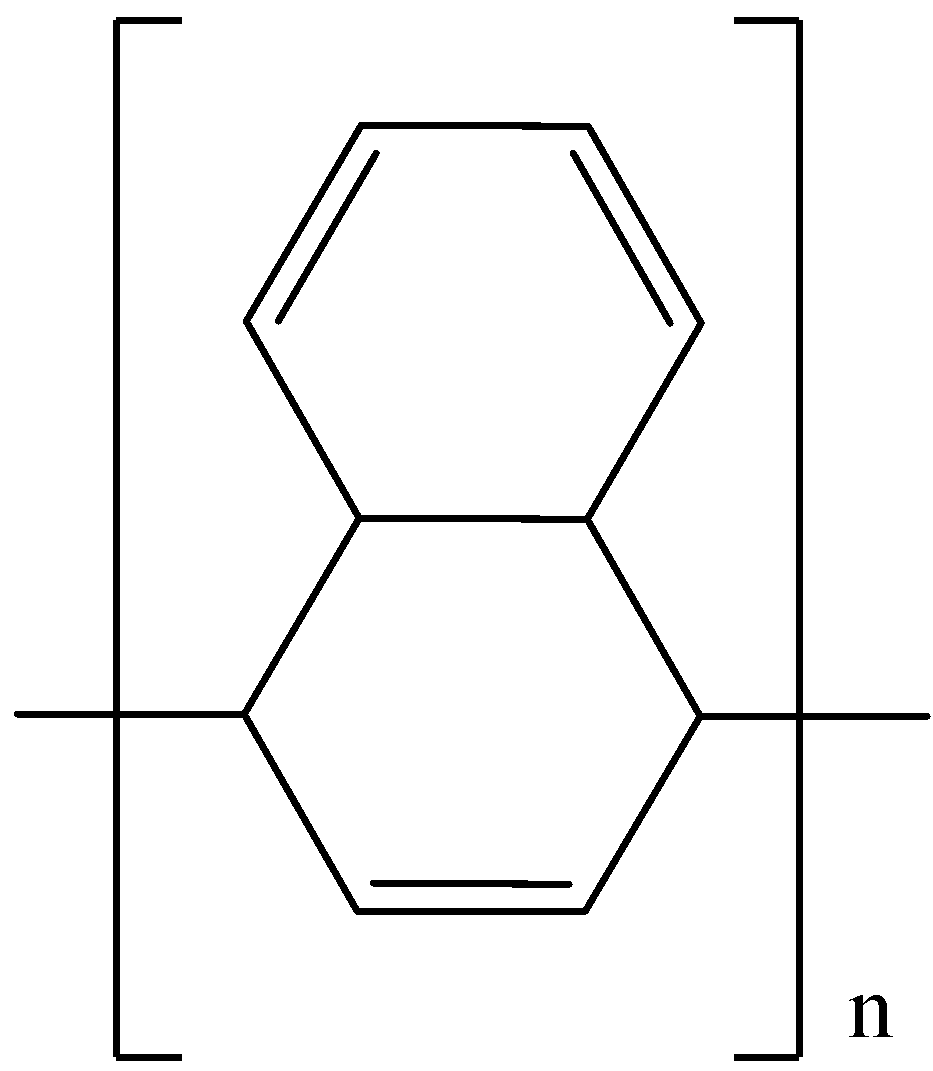
Solution
. In Kolbe electrolysis the potassium salts of the carboxylic acids undergo decarboxylation and form free radicals. The formed free radicals from the carboxylic salts undergo dimerization and form symmetrical dimers or polymer products.
Complete step by step answer:
- In the question it is given that potassium salt of carboxylic acid undergoes electrolysis.
- We have to find the product A in the given reaction.
- The reaction is as follows.
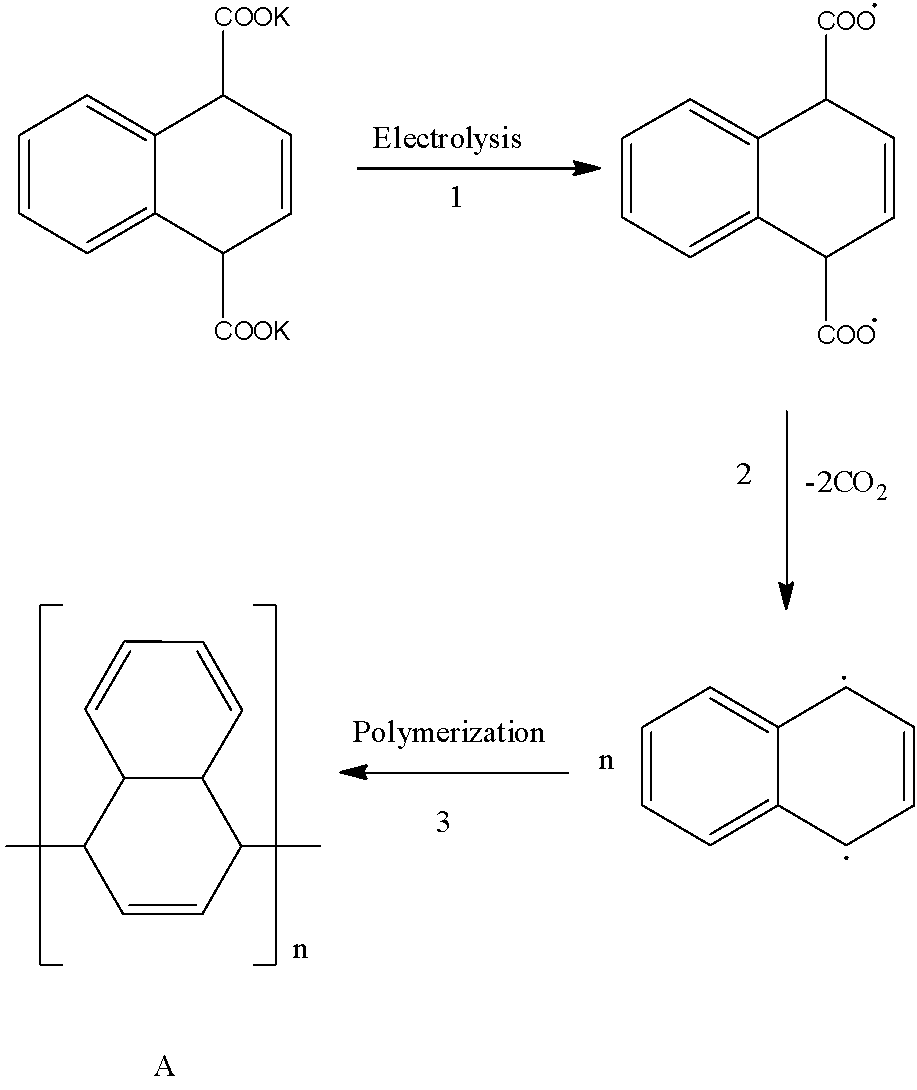
- In the first step the given compound containing potassium salt of dicarboxylic acid releases potassium in the form of free radicals and forms dicarboxylic free radicals as the product.
- The product formed in the first step the formed dicarboxylic free radicals product undergoes decarboxylation and forms a compound having two free radicals on the ring at two different places.
- The product formed in the second step undergoes polymerization and forms the product A as the major product in the third step.
- Therefore the given organic compound containing two potassium salts of carboxylic acid undergoes Kolbe electrolysis and forms a polymerized derivative as the product.
So, the correct answer is “Option D”.
Note: Generally in organic chemistry Kolbe electrolysis is used to prepare symmetrical dimers by using potassium salt of carboxylic acid through free radical mechanism. The Kolbe reaction is also called a decarboxylative dimerization reaction. To undergo Kolbe electrolysis the organic compound should contain two carboxylic functional groups at two different positions in its structure.
