Question
Question: The compound 1, 2-butadiene has: A. Only sp hybridised carbon atoms B. Only \[s{p^2}\] hybridise...
The compound 1, 2-butadiene has:
A. Only sp hybridised carbon atoms
B. Only sp2 hybridised carbon atoms
C. Both sp and sp2 hybridised carbon atoms
D. sp, sp2 and sp3 hybridised carbon atoms
Solution
We know that the phenomenon of mixing of orbitals of the same atom with slight difference in energies so as to redistribute the energies and give new orbitals of equivalent energy and shape is termed as hybridization.
Complete step-by-step solution:
Here we first find the hybridisation of each carbon individually either it is sp2, sp3 or sp.
sp3 hybridization uses four sp3 hybridized atomic orbitals. So, there must be the presence of four groups of electrons.
sp2 hybridization uses three sp2 hybridized atomic orbitals. So, there must be the presence of three groups of electrons.
sp hybridization uses two sp hybridized atomic orbitals. So, there must be the presence of three groups of electrons.
Let’s come to the question. The structure of 1, 2-butadiene is as follows:
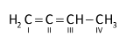
,the 1st carbon is sp2 hybridized as three electrons groups present, 2nd carbon is sp hybridised (two electron groups) , 3rd carbon is sp2 hybridized (three electrons groups) and 4th carbon is sp3 hybridized because of presence of four electron groups.
Therefore, in 1, 2-butadiene, sp2, sp and sp3 carbons are present. Hence, D is the correct option.
Note: Hybridisation of carbon atom can be found by counting the number of electron groups surrounding the carbon atom. If four groups present such as in case of CH4 the hybridization is sp3. If three electron groups are present, hybridization is sp2 and if two electron groups present, hybridization is sp.
