Question
Question: The complex salt \(CoCl_{ 4 }^{ 2- }\) has a tetrahedral structure. How many d-electrons are on the ...
The complex salt CoCl42− has a tetrahedral structure. How many d-electrons are on the cobalt?
Solution
Hint: First just try to find the hybridization of the complex and then you can just calculate the electrons present. But keep in mind that if there is some charge left on the metal atom you have to satisfy that also. Now, you can easily solve this question.
Complete step by step answer:
First, we know that it is a tetrahedral complex so there is only one possibility of the hybridization of the molecule. That would be sp3.
In sp3 hybridization, we need one s and three p hybrid orbitals. These should be degenerate.
You should know that here Cl− is a weak ligand, hence pairing is not possible.
Co has the atomic number 27, which means it has 3d74s2 electronic configuration.
In CoCl42− complex, if we calculate the oxidation number of copper, it would be-
x + 4(-1) = -2
x = +2
So, here copper is in +2 oxidation state. That means it loses 2 electrons from 4s orbital.
After losing 2 electrons it is left with only 7 electrons and those are located in 3d orbitals.
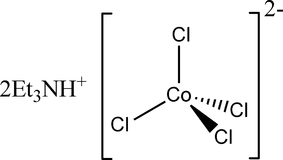
Therefore we can say that there are 7 d-electrons on the cobalt in complex salt CoCl42−.
Note: The geometry of sp3 Hybridization:
sp3 hybridized orbitals repel each other and they are directed to four corners of a regular tetrahedron. The angle between them is 109.5 degrees and the geometry of the molecule is tetrahedral (non-planar). This type of hybridization is also known as tetrahedral hybridization.
