Question
Question: The complex \( \left[ {Ni{{\left( {dmg} \right)}_2}} \right] \) , (where \( dmg \) is dimethylglyoxi...
The complex [Ni(dmg)2] , (where dmg is dimethylglyoxime):
(A) Has a square planar geometry and is paramagnetic in nature
(B) Has a square planar geometry and is diamagnetic in nature
(C) Has a tetrahedral geometry and is paramagnetic in nature
(D) Has a tetrahedral geometry and is diamagnetic in nature
Solution
to determine the shape of the complex we have to first look at the ligand. If the given ligand is strong then it will pair the unpaired electrons of the element whereas if the given ligand is weak there will be no change in the electrons of the element.
Complete answer:
To answer this question we have to write the electronic configuration of nickel then we will write the electronic configuration of nickel (II) ion then we will write the electronic configuration of [Ni(dmg)2] which are as follows:
Ni=3d84s2
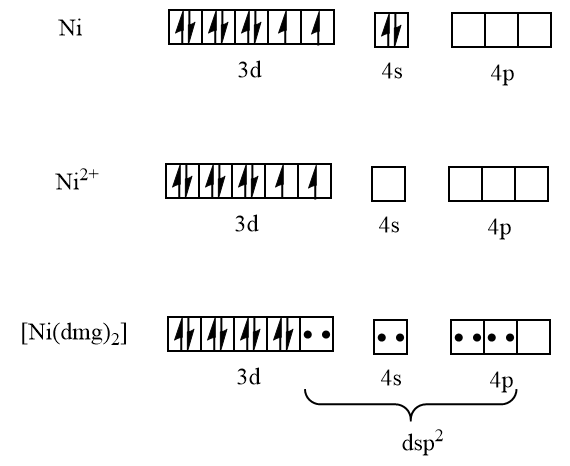
Here the coordination number is four and it has dsp2 hybridization. From the diagram it is clear that all electrons here are paired thus it is diamagnetic in nature. And nickel with coordination number four and dsp2 hybridization has the square planar geometry which is:
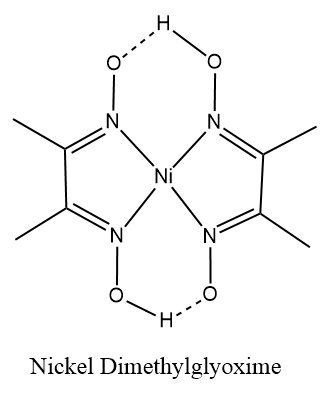
Thus, the complex [Ni(dmg)2] has square planar geometry and is diamagnetic in nature.
Therefore, option (b) is correct.
Note:
Here the dmg which is dimethylglyoxime, is a polydentate ligand. The polydentate ligands are those in which the numbers of donor atoms are two or more than two atoms. They form bonds to a central metal atom or ion. In other words they are the ligands which are attached with the central metal ion or atom by bonds from two or more donor atoms.
