Question
Question: The complex ion \( {[Cu{(N{H_3})_4}]^{2 + }} \) is: (A) Tetrahedral and paramagnetic (B) Tetrahe...
The complex ion [Cu(NH3)4]2+ is:
(A) Tetrahedral and paramagnetic
(B) Tetrahedral and diamagnetic
(C) Square planar and paramagnetic
(D) Square planar and diamagnetic
Solution
Hint : We will use the concept of Crystal field theory which states that the breaking of orbital degeneracy in transition metal complexes happens due to the presence of Ligands. On the basis of hybridization, we will explain the geometry of [Cu(NH3)4]2+ and also by determining the nature of the ligand according to its position in spectrochemical series i.e. whether it is a strong ligand or a weak ligand.
Complete Step By Step Answer:
As we know the atomic number of Cu is 29 so the electronic configuration will be:- 1s22s23s23p64s23d9
But here Cu exists in Cu+2 oxidation state so two electrons will be eliminated from the 4s orbital so the electronic configuration of Cu+2 will be:- 1s22s23s23p63d94s0
We will now draw the orbitals of 3d,4s&4p to determine the hybridization: -
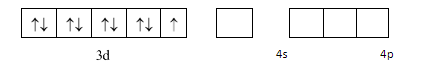
As NH3 is a strong ligand so pairing should take place but here we see that an electron is transferred from 3d orbital to 4p orbital . This happens according to the concept of transference which states that an electron can be transferred from a lower energy orbital to a higher energy orbital if strong ligands are present and if there is a possibility of formation of a lower orbital complex. We depict it as: -
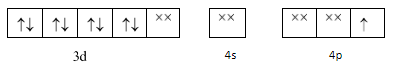
Now these dsp2 hybrid orbitals will accept the electron pair from the four NH3 ligands individually and thus will form a dsp2 hybridization and a square planar geometry.
As the compound contains 1 unpaired electron so the magnetic nature would be paramagnetic.
So, the correct option is Option c i.e. Square planar and paramagnetic.
Note :
Practically [Cu(NH3)4]2+ should form sp3 hybridization with tetrahedral geometry but this does not happen because 3d orbital contains only one unpaired electron, so the unpaired electron is shifted to 4p orbital for more stability. This cause of transference and the source of energy required for transfer of electrons could not be explained by the Valence Bond Theory.
