Question
Question: The colour of the final product ‘D’ is: 
A.Deep blue
B.Deep orange
C.White
D.Pink
Solution
To solve this question, we must proceed with one reaction at a time and proceed with taking into consideration each chemical species and its properties that are being used. The given compound in the question is benzene which undergoes Sandmeyer’s reaction to form diazonium salt.
Complete step by step answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Benzene is an aromatic compound and the reaction of benzene with dinitrogen pentoxide or N2O5 to form a NO2 based compound of benzene. The name of this compound is nitrobenzene. Dinitrogen pentoxide causes the nitration of benzene. The chemical reaction for the same can be given as:
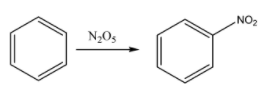
Now, this nitrobenzene is made to react with tin in the presence of hydrochloric acid or HCl. This will result in the substitution of the oxygen atom from the NO2 substituent and result in the formation of an amino functional group. When amine is attached directly to the benzene ring, then that compound is commonly known as aniline. The chemical reaction for the same can be given as:
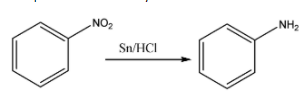
Now, Aniline is made to react with sodium nitrate and HCL. This causes the formation of a compound named benzene diazonium chloride. The chemical reaction for the same can be given as:
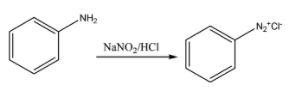
Now, finally, this benzene diazonium chloride is made to react with beta- naphthol. This beta – naphthol couples with the phenyl diazonium electrophile to produce an intense orange-red dye. The chemical reaction for the same can be given as:
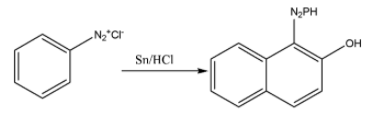
Hence, Option B is the correct option
Note:
The orange – yellow dye thus formed is known as Solvent yellow 7. The compound formed is a yellowish orange coloured azo compound, and this entire reaction is base catalysed. Another common name for this compound is aniline yellow.
