Question
Question: The color and magnetic nature of manganite ion (\(MnO_4^{2 - }\)) are: A.Green, paramagnetic B....
The color and magnetic nature of manganite ion (MnO42−) are:
A.Green, paramagnetic
B.Purple, diamagnetic
C.Green, diamagnetic
D.Purple, paramagnetic
Solution
As we know the color and magnetic behavior of an ion can be determined with the help of unpaired electrons and if there is no unpaired electron then it is diamagnetic and if it contains unpaired electrons then it is paramagnetic
Complete step by step answer:
As we know manganese (Mn) is a d-block element having an atomic number 25 so its outer electronic configuration is 3d54s2. The configuration is shown in the diagram below
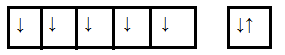
To find the unpaired electrons first, we have to find the oxidation state of Mn in the case of manganite ions. Let us consider the oxidation state of Mn ion in the above case is x. and we know the oxidation state of oxygen is -2. So,
x+4×(−2)=−2 ⇒x−8=−2 ⇒x=8−2 ⇒x=6
In the +6 oxidation state, the outer configuration of Mn will be 3d1.
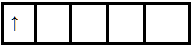
Hence there is one unpaired electron so it is paramagnetic. And due to the presence of an unpaired electron, it is purple.
So, the correct answer is Option D.
Note:
We can explain the color and magnetic properties of a compound with crystal field splitting theory. In this theory, degenerate orbitals split into higher energy levels and lower energy levels. When an electron absorbs radiation it gets to jump to the higher energy level where it becomes unstable and returns to a lower level by releasing the same amount of energy that is absorbed as a result it shows color.
