Question
Question: The coefficient of linear expansion of a cubical crystal along three mutually perpendicular directio...
The coefficient of linear expansion of a cubical crystal along three mutually perpendicular directions is α1 α2 and α3 . What is the coefficient of cubical expansion of the crystal?
Solution
You may start with a cuboid and set the temperature to rise from 0∘C to T∘C as a first step. Then you might find the linear expansion equation in all three dimensions. The volume of the solid will then be determined by taking their product. You can get an expression by expanding that expression and then ignoring the smaller terms. You may figure out the answer by comparing it to the volume expansion expression.
Complete answer:
Considering a cubical crystal structure
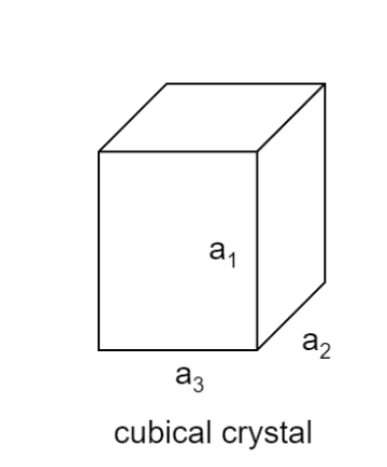
Let α1 α2 and α3 . be the coefficient of linear expression along the sides is a1 a2 and a3.
We know for linear expansion
a=a0(1+αΔT)
Where a0 is the initial edge length
Take the temperature of the solid above to rise from 0∘C to T∘C
ΔT=T−0=T
So for all the edges we have
a1′=a1(1+α1T)
a2′=a2(1+α2T)
a3′=a3(1+α3T)
After expansion, their product will give the volume of the cuboid at temperature T∘C .
V′=a1′a2′a3′=a1a2a3(1+α1T)(1+α2T)(1+α3T)
However, we know that the cuboid's initial volume is determined by the product of the edges. That is to say,
V0=a1a2a3
By neglecting small values from the above equation we get
V′≈V0(1+(α1+α2+α3)T)
Now using the formula of volume expansion
V=V0(1+γΔT)
Comparing this from the above equation we get
γ=α1+α2+α3
Note:
Thermal expansion is the phrase used to describe the tendency of matter to change in length, area, and volume as a result of temperature changes. When molecules are heated, they move and vibrate more, increasing the space between them. The ratio of relative expansion to temperature change can be characterised as the coefficient of thermal expansion.
