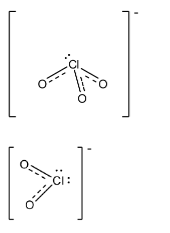
Question
Question: The chlorine to oxygen bond distance in \(ClO_{4}^{-}\) is 1.44 \(\overset{\circ }{\mathop{\text{A}}...
The chlorine to oxygen bond distance in ClO4− is 1.44 A∘. From this, we can conclude that there must be a considerable double bond character in the bonds.
If the given statement is true then enter 1, else enter 0.
Solution
In ClO4−, the central atom is chlorine and there are four oxygen atoms joined to it. There are seven electrons in the valence shell of chlorine and it has one negative charge, so there was an addition of one electron in the compound. And one oxygen atom is attached through two electrons of the chlorine atom.
Complete step by step solution: In ClO4−, the central atom is chlorine and there are four oxygen atoms joined to it. There are seven electrons in the valence shell of chlorine and it has one negative charge, so there was an addition of one electron in the compound. Generally the length of the single bond between chlorine and oxygen is 1.69 A∘ and the length of the double between chlorine and oxygen is 1.40 A∘. In ClO4−, one oxygen atom is attached through two electrons of the chlorine atom, so in this the length of all the bonds present in the compound is 1.44 A∘. The structure is given below:
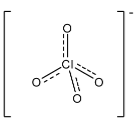
As we can see that there is a partial double bond in all the bonds in the compound, and the bond is near to the bond length of the double bond. Therefore, it is considered a double bond.
Hence, the given statement in the question is true.
Note: In ClO3− and ClO2−, there is also partial double bond character, as in ClO4− and there bond length is also the same as in the ClO4−. The structures of both the compounds are given below: