Question
Question: The chirality of the compound is 
A. R
B. S
C.Z
D. E
Solution
As we know that stereochemistry is the investigation of the three‐dimensional design of particles. The cis and trans isomers are types of stereoisomers, varying fundamentally just in the area of the particles of the atom in three‐dimensional space. Such stereoisomers can have distinctive physical and substance properties. For instance, the cis and trans isomers of butenedioic corrosive show tremendous contrasts in their physical and compound properties.
Complete answer:
We have to know that the "right hand" and "left hand" classification is utilized to name the enantiomers of a chiral compound. In the event that the bolt focuses in a counterclockwise way, the design at stereo-center is viewed as S.
We have to know that, at the letter E, the level strokes are largely on a similar side; in the E isomer, the higher need bunches are on inverse sides. In the letter Z, the even strokes are on inverse sides; in the Z isomer, the gatherings are on a similar side.
Least needed molecule is in every case away from the watcher. Need is seen based on nuclear no. furthermore, if nuclear no. are same then based on nuclear mass. Find in the request for higher to bring down need.
The structure of stereochemistry of the given compound is given,
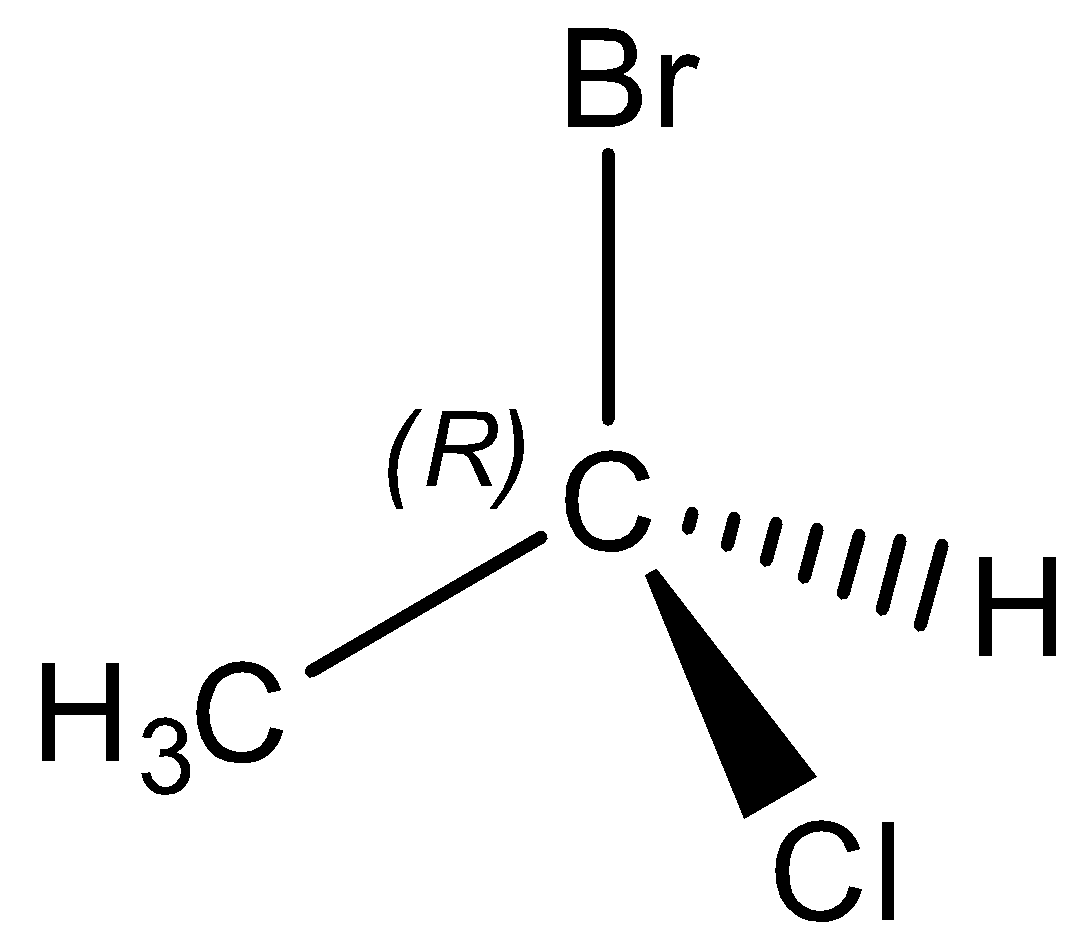
On the off chance that clockwise, it is R.
On the off chance that anticlockwise, it is S.
Complete name of the particle is (R) 1 - bromo-1 - chloroethane.
Hence, the correct option is (A).
Note: We have to see, the utilization of single-enantiomer medications can conceivably prompt easier and more particular pharmacologic profiles, improved helpful files, less complex pharmacokinetics because of various paces of digestion of the various enantiomers, and diminished medication associations.
