Question
Question: The chemical name of ‘Aspirin’ is: a. Benzyl salicylate b. Acetyl salicylic acid c. Acetyl cya...
The chemical name of ‘Aspirin’ is:
a. Benzyl salicylate
b. Acetyl salicylic acid
c. Acetyl cyanamide
d. Tartaric acid
Solution
Hint: Aspirin is one of the most commonly analgesic and antipyretic medications worldwide, being in use for over 100 years. It is also known as acetosal.
Complete answer:
As the name acetosal suggests, the chemical name of Aspirin is Acetyl salicylic acid. Aspirin is an analgesic used to reduce pain, fever or inflammation. Aspirin given shortly after heart attack reduces the risk of a heart attack. It is also used in the long term to prevent heart attacks and blood clots. Certain types of cancer, mainly colorectal cancer can also be prevented by Aspirin. Aspirin is a nonsteroidal anti- inflammatory drug (NSAID). Aspirin is also one of the most commonly used drugs for migraines. In high doses, it can treat or reduce rheumatic fever, rheumatic arthritis and pericarditis. Its chemical formula is H8C9O4
The structure of Aspirin is:
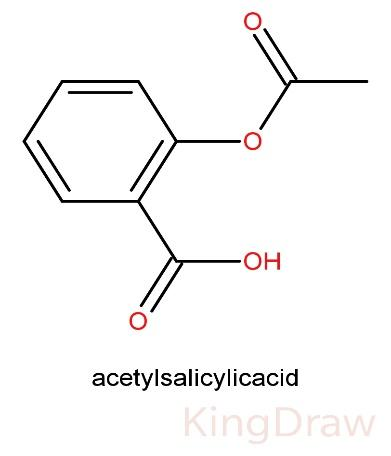
Salicylic acid is acetylated using acetic anhydride, yielding aspirin and acetic acid as by-products. Practically, its yield is generally low due to the relative difficulty of its extraction from aqueous state.
The chemical reaction involved in the synthesis of Acetyl salicylic acid is:
C7H6O3+C4H6O3→H8C9O4+C2H4O2
C7H6O3 is salicylic acid.
C4H6O3 is acetic anhydride.
C2H4O2 is acetic acid.
While with its numerous benefits, it also has some side effects. Most common side effects are:
- Upset stomach
- Stomach ulcers
- Stomach bleeding
- Asthma
- Headache
- Nausea
- Drowsiness
People with alcohol habits, who are older, take other NSAIDs are at a higher risk of bleeding. It is also not recommended in children with infections due to the high risk of Reye syndrome.
Note: The structure of salicylic acid should be known to get the structure or formula of Aspirin. Else, it would be a hard time getting the formula of Aspirin.
