Question
Question: The chemical formula of the compound containing carbon and oxygen is: A. CO B. \({\text{C}}{{\te...
The chemical formula of the compound containing carbon and oxygen is:
A. CO
B. CO2
C. CO3
D. Both A and B
Solution
To answer this question we should know the maximum valency shown by carbon and oxygen. The maximum valency shown by carbon is four. The maximum valency shown by oxygen is two. So, carbon can make four maximum bonds. We will take the carbon and oxygen and will check that in that ratio the compound can form or not.
Complete answer:
The maximum oxidation state shown by carbon is +4 and it can also be +2. The maximum oxidation state shown by oxygen is +2.
Let’s take the 1:1 combination of carbon and oxygen and check the compound can form or not as follows:
The compound formed by 1:1 combination of carbon and oxygen is CO. CO is known as carbon monoxide.
The structure of CO is shown as follows:
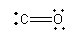
Here, carbon and oxygen both are in +2 oxidation state. The valency of both carbon and oxygen is also satisfied, so the compound formed by carbon and oxygen can have chemical formula CO.
Let’s take the 1:2 combination of carbon and oxygen and check the compound can form or not as follows:
The compound formed by 1:2 combination of carbon and oxygen is CO2. CO2 is known as carbon dioxide.
The structure of CO2 is shown as follows:
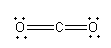
Here, carbon is in +4and oxygen is in +2 oxidation state. The valency of both carbon and oxygen is also satisfied so, the compound formed by carbon and oxygen can have chemical formulaCO2.
Let’s take the 1:3 combination of carbon and oxygen and check the compound can form or not as follows:
The compound formed by 1:3 combination of carbon and oxygen is CO3. CO3 is known as carbon trioxide.
The structure of CO3 is shown as follows:
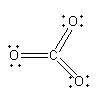
Here, carbon is in +6and oxygen is in +2 oxidation state. The valency of carbon is exceeding and oxidation state is also exceeding form the maximum oxidation state shown by carbon so, compound formed by carbon and oxygen cannot have chemical formulaCO3.
So, the chemical formula of the compound containing carbon and oxygen is CO and CO2.
Therefore, option (D) Both A and B is correct.
Note: Maximum valency four means that carbon and form four sigma bonds or it can form two sigma and two pi bonds. One other possibility is that carbon can form one sigma and one pi bond and can save its remaining electrons as lone pairs. Oxygen can form two sigma bonds or one sigma one pi bond. In CO3, if the carbon can show maximum oxidation state +4 so, if two oxygen combines with carbon in −1 state and one oxygen combines in −2 then the +4 charge of carbon will be satisfied by −4 charge of three oxygen and a compound of one carbon and three oxygen can be formed which will have chemical formula as CO32− .
