Question
Question: The chemical formula of calcium hypochlorite is: (A) \(COC{{l}_{2}}\) (B) \(Ca{{(ClO)}_{2}}\) ...
The chemical formula of calcium hypochlorite is:
(A) COCl2
(B) Ca(ClO)2
(C) Ca(ClO3)2
(D) Ca(ClO4)2
Solution
in calcium hypochlorite, calcium is bonded to two oxygen atoms and oxygen atom is linked to chlorine atom and calcium. Oxidation number of calcium is +2. Oxygen has oxidation state as -2 and chlorine has oxidation state as +1.
Complete solution step by step:
-In bleaching powder, chlorine powder or chlorinated lime, calcium hypochlorite is the main active ingredient.
-in calcium hypochlorite, calcium is bonded to two oxygen atoms and oxygen atom is linked to chlorine atom and calcium. Oxidation number of calcium is +2. Oxygen has oxidation state as -2 and chlorine has oxidation state as +1.
-structure of calcium hypochlorite is as follows:
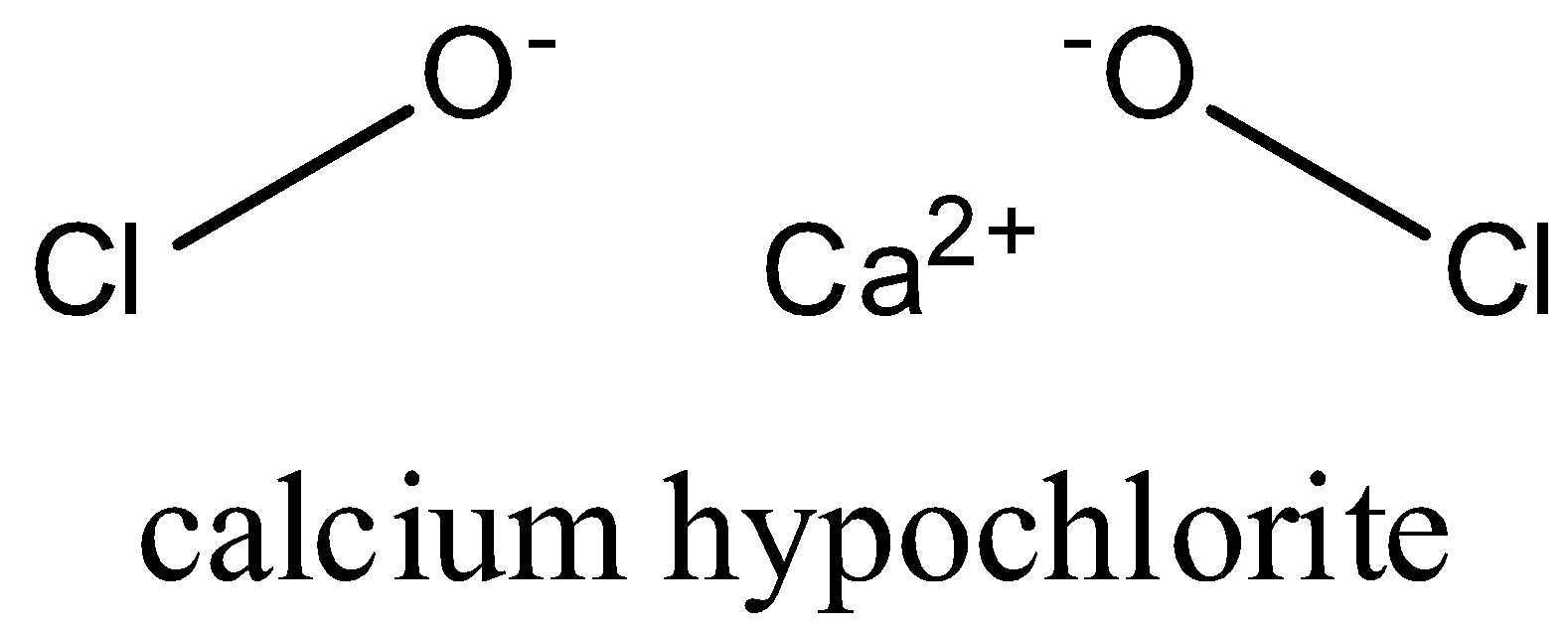
-it is used in water treatment to remove hardness of water and also as a bleaching agent.
-it is an inorganic compound as there is no carbon involved in structure.
- it is more stable and easily available.
-it has a strong smell of chlorine and it decomposes in moist air.
-it has good solubility in soft to medium hard water.
-it is prepared by treating calcium hydroxide known as lime with chlorine gas.
It exists in two forms, dry and hydrated.
-it is commonly used for sanitization of swimming pools. It is a good oxidizing agent.
The chemical formula of calcium hypochlorite is (B) Ca(ClO)2
Note: Calcium hypochlorite is basic in nature. It is used confused with calcium oxychloride. Oxidation number of calcium is +2. Oxygen has oxidation state as -2 and chlorine has oxidation state as +1. It is used as a disinfectant. COCl2 is carbonyl chloride.
