Question
Question: The CFSE for \({\left[ {{\text{CoC}}{{\text{l}}_6}} \right]^{4 - }}\) complex is \(18000\,{\text{c}}...
The CFSE for [CoCl6]4− complex is 18000cm−1. The for [CoCl4]2− will be
A. 18000cm−1
B. 16000cm−1
C. 6000cm−1
D. 2000cm−1
Solution
The octahedral crystal field splitting is equal to the 4/9 of octahedral crystal field splitting. The octahedral crystal field splitting is larger than the tetrahedral crystal field splitting.
The following formula can be used-
td = 94 oh
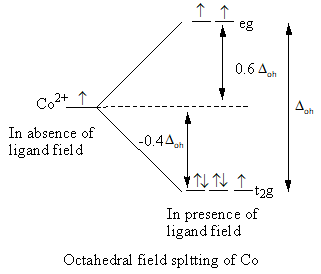
Step by step answer: he octahedral field splitting is represented as follows:
Valence electronic configuration of Co2+ = 3d7
The tetrahedral field splitting is represented as follows:
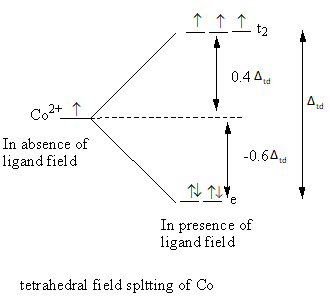
The relation between the energy difference of tetrahedral and octahedral field is as follows:
td = 94 oh
Where,
tdis the tetrahedral field splitting energy.
oh is the octahedral field splitting energy.
In the octahedral complex, six ligands split the energy level of the metal and the tetrahedral complex four ligands split the energy level of the metal, so the value of octahedral crystal field splitting is larger than the tetrahedral crystal field splitting.
Substitute 18000cm−1 for oh.
td = 94×18000cm−1
td = 8000cm−1
So, the tetrahedral field splitting energy for [CoCl4]2−complex is 8000cm−1.
So, option (A), (B) and (D) are incorrect.
Therefore, option (C) 8000cm−1 is correct.
Additional information: The oh per ligand is determined by dividing the oh by six and td per ligand is determined by dividing the td by four.
Note: Both the values for octahedral as well as tetrahedral should be in the same unit. As the octahedral crystal field splitting is always larger than the tetrahedral crystal field splitting so, here, the answer of td will be less than18000cm−1. For the calculation of oh from the given td, the formula used will be oh = 49 td.
