Question
Question: The central oxygen atom in ether is ----...
The central oxygen atom in ether is ----
Solution
In ether molecules one atom of oxygen is combined with two groups of alkyl or aryl groups. On the basis of the nature of substituents adjacent to oxygen atoms they are categorized as symmetrical ether or unsymmetrical ether.
Complete answer:
Electronic configuration of oxygen is 1s22s22px22py12pz1 and having two unpaired electrons present in its p-orbital.
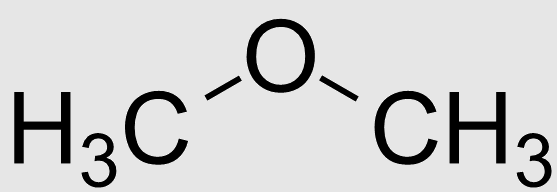
Let the ether compound is dimethyl ether with molecular formula CH3OCH3
In ether moiety central oxygen forms a bond with the carbon atom of alkyl group.
Four valence orbital of oxygen atom [2s,2px,2py,2pz] undergoes hybridization to form four orbitals of equal energy (sp3).
Two hydrogen atoms of the alkyl group form a bond with [2py1,2pz1] to form a sigma bond .
Hence two lone pair electrons are still present in the oxygen atom in its [2s2,2p2] orbitals.
These two lone pairs arrange themselves at a particular angle to form a stable structure.
These lone pair pushed the bond between oxygen and hydrogen (O−H) toward each other and reduces the bond angle to (104.5∘) and acquired the final shape as Bent or V-shaped molecule.
Hence from the above discussion we see that the central oxygen atom in ether is sp3 hybridized with two lone pairs of electrons.
Note:
Other molecules shows sp3 hybridization includes- CH4,SnCl4,SiCl4,SiF4,NH4+ etc.
Regular geometry of sp3 hybridization is tetrahedral, but it may vary due the presence of lone pairs of electrons in the compound.
