Question
Question: The cationic part of the solid \(Xe{{F}_{6}}\) is having the ‘______’ shape. (a)- Linear (b)- An...
The cationic part of the solid XeF6 is having the ‘______’ shape.
(a)- Linear
(b)- Angular
(c)- Square pyramidal
(d)- Tetrahedral
Solution
The cationic part of XeF6 is based on the number of atoms joined to the central atom. Not only atoms, but lone pairs are also considered for the shape. For finding the shape of the compound the number of the total number of electron pairs and the number of lone pairs should be calculated with the help of valence electrons, the number of bonds, etc.
Complete answer:
The reaction of XeF6 to form cation and anion is:
XeF6→XeF5++F−
So, we have to find the structure of XeF5+.
Both the VSEPR theory and the concept of hybridization are applied to predict the molecular geometries of xenon compounds.
According to the VSEPR theory, the shape of the molecule is predicted by the total number of electron pairs (lone pairs + bond pairs) in the valence shell of the central Xe atom.
To calculate the total number of electron pairs:
2valence electrons of central atom + number of bonded atoms
Since 5 fluorine atoms are bonded to the xenon atom and the molecule has a positive charge hence one electron will be reduced.
With the above formula: 28+(5−1)=28+4=6
Hence, there are 6 electron pairs.
Since there are 5 fluorine atoms attached to xenon, there are 5 bond pairs of electrons.
Now for calculating the number of lone pairs in the compound: -
Total number of electron pairs –number of bond pairs.
Lone pairs = 6 – 5 = 1
Hence, in the compound, there are 1 lone pair.
Depending on the number of Xe−F covalent bonds to be formed, the requisite number of electrons of the 5p−orbital valence shell of Xe gets unpaired and promoted to the vacant 5d−orbitals followed by hybridization.
Since there are 5 bond pairs of an electron and one lone pair hence the hybridization is sp3d2.
So, the hybridization is sp3d2 and has one lone pair the structure of XeF5+ is square pyramidal.
The structure is given below:
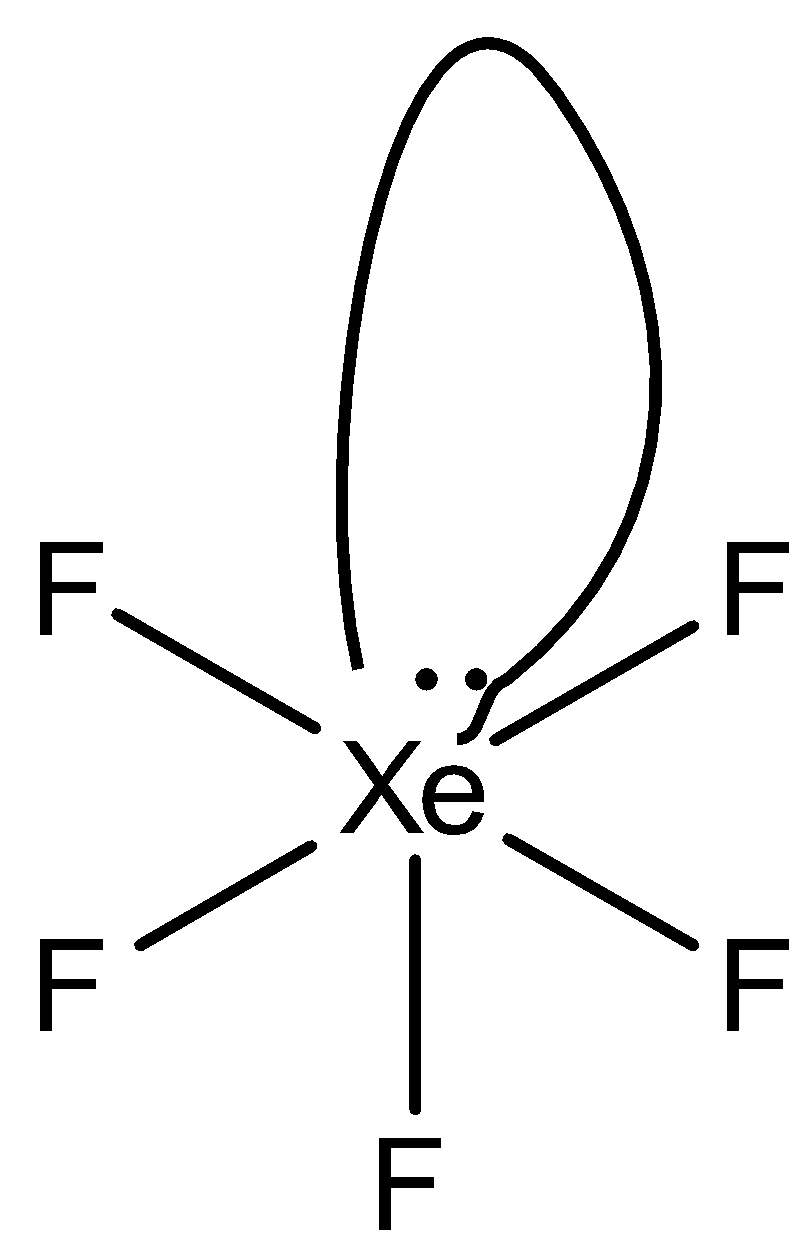
So, the correct answer is “Option C”.
Note: Whenever you are drawing the compound structure the number of lone pairs should also be considered. For example, when 4 atoms of fluorine are bonded with xenon you could get confused between tetrahedral, square planar shape, and Trigonal bipyramidal.
