Question
Question: The calculated bond order of superoxide ion \[\left( {{\text{O}}_{\text{2}}}^{-} \right)\]is: a.) ...
The calculated bond order of superoxide ion (O2−)is:
a.) 2.5
b.) 2
c.) 1.5
d.) 1
Solution
Hint: To answer this question, we should know about bond order. We should know the steps to find out the bond order.
Complete step by step solution:
First of all, we will know bond order. We should know that bond order is the number of chemical bonds between a pair of atoms and indicates the stability of a bond. If we take an example of diatomic nitrogen, N≡N , the bond order is 3.
We should know that bond order is the number of bonding pairs of electrons between two atoms. Let us take the example of a covalent bond, in a covalent bond that occurred between two atoms, a single bond has a bond order of one, a double bond has a bond order of two, a triple bond has a bond order of three, and so on.
We have to follow some steps in order to find the bond order. These steps are:
Let us first discuss superoxide ion and then we will draw its molecular orbital diagram.
We should know that a superoxide is a compound that contains the superoxide ion, which has the chemical formula O−. We should know that superoxide is the anionic form O2−. It is important as the product of the one-electron reduction of di-oxygen (oxygen gas), which occurs widely in nature. With one unpaired electron, the superoxide ion is a free radical.
Let us draw the molecular orbital diagram of superoxide ion of oxygen.
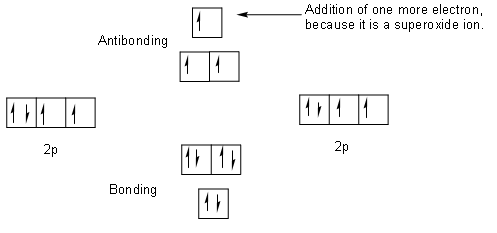
The above figure is the molecular orbital diagram of superoxide ion. We first draw the molecular orbital diagram of oxygen and then we will add one electron at an anti-bonding site to make it a molecular diagram of superoxide ion.
We should know the formula to calculate bond order of molecules by observing molecular orbital theory.
