Question
Question: The C – O bond length in \(CO\),\(C{O_2}\) and \(CO_3^{2 - }\) follows the order A) \(CO < C{O_2} ...
The C – O bond length in CO,CO2 and CO32− follows the order
A) CO<CO2<CO32−
B) CO>CO2>CO32−
C) CO2<CO32−<CO
D) CO32−<CO2<CO
Solution
The type of bond between two atoms gives the relative measure of the bond length. We can determine the type of bond by simply drawing the Lewis dot structure. Stronger is the bond smaller is the bond length.
Complete step by step answer:
The order of C – O bond length in CO, CO2 and CO32−.
Firstly, we need to determine the type of bond in each of the molecules. Triple bond is the strongest and the shortest bond while, single bond is the longest and the weakest.
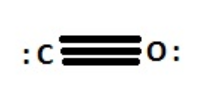
Lewis dot structure of CO shows triple bond between the carbon and the oxygen.
While, for CO2, the Lewis structure gives one double bond each oxygen and carbon atom.
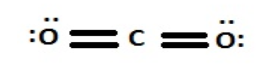
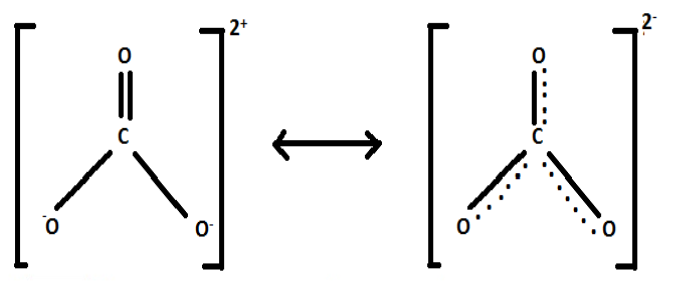
In CO32−, the Lewis dot structure gives one double bond and two single bonds between C and O. Due to resonance, the single bond has partial double bond character. Thus, the bonds in CO32−are shorter than a single bond but longer than a double bond.
Hence, the increasing order of bond length –
CO<CO2<CO32−
Option (A) is the correct option.
Note:
Students might also use an alternative method by calculating the bond orders for the molecule. As bond order is inversely proportional to bond length, so, we can compare bond length in terms of values of bond order –
CO(B.O=3.5)<CO2(B.O=2)<CO32−(B.O=1.33)
It is important to note that the value of bond order is calculated with the help of Molecular Orbital (M.O) theory using M.O diagram.
