Question
Question: The \[C{O_2}\] laser where sunlight shines on the atmosphere of Mars, carbon dioxide molecules at an...
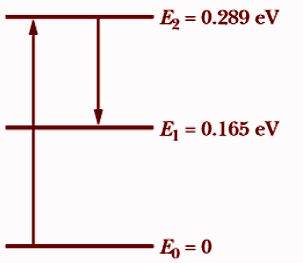
The CO2 laser where sunlight shines on the atmosphere of Mars, carbon dioxide molecules at an altitude of about 75km undergo natural laser action. The energy levels involved in the action are shown in the above figure; population inversion occurs between energy levels E2 and E1.
(a) What wavelength of sunlight excites the molecules in the lasing action?
(b) At what wave-length does aliasing occur?
(c) In what region of the electromagnetic spectrum do the excitation and lasing wavelengths lie?
Solution
One of the first gas lasers to be developed was the carbon dioxide laser. Kumar Patel of Bell Labs invented it in 1964, and it is now one of the most useful forms of laser. Carbon dioxide lasers are now the most powerful continuous wave lasers available. They're also very efficient: the output power to pump power ratio can be as high as 20%. The CO2 laser generates an infrared light beam with wavelength bands centred on 9.6 and 10.6 micrometres (μm).
Complete step by step answer:
(a) As the electron jumps from the peak energy level E2 to the lowest level E1, the laser operation occurs. As a result, the wavelength that corresponds will satisfy.Change in electron energy is given as,
ΔE=E2−E1
⇒ΔE=E2−E1=λhc
This can be rewritten as
λ′=E2−E1hc
⇒λ′=0.289eV−0.165eV1240eV⋅nm
⇒λ′=1.00×104nm
∴λ′=10.0μm
(b) At the wavelength of 0.4 & 0.8 micrometer aliasing will occur.
(c) So, the wavelength lies in the visible region.
Note: The visible light continuum is the portion of the electromagnetic spectrum that can be seen by the naked eye. This spectrum of wavelengths is referred to as visible light.The human eye can sense wavelengths ranging from 380 to 700 nanometers in most cases.Visible light has wavelengths ranging from 400 to 750 nanometers (0.4 to 0.75 micrometres).Human eyes are only open to this part of the continuum. In the visible part of the spectrum, the Sun emits the most radiation.
