Question
Question: The bonds present in borazine or inorganic benzene are: A. \(12\sigma ,~3\pi \) B. \(9\sigma ,~6...
The bonds present in borazine or inorganic benzene are:
A. 12σ, 3π
B. 9σ, 6π
C. 6σ, 6π
D. 9σ, 9π
Solution
Hint: To find the number of σ (Sigma) and π (Pi) bonds in a compound we must know the basic idea behind them. Every bond has only and necessarily 1σ bond. So, a single bond consists of 1σ bond. The double bond consists of 1σ bond and 1π bond. Theσ bond is formed by the head-on overlap of two sp2 orbital. Theπ bond is formed by the side-on overlap of two 2p orbital. A triple bond contains 1σ bond and 2π bonds.
Complete answer:
Sigma and Pi bonds are covalent chemical bonds. These bonds are formed by the overlap of atomic orbital. Sigma bonds are formed by end-to-end or head on overlapping and Pi bonds are when the lobe of one atomic orbital overlaps another or sideways overlapping. The given compound borazole or borazine also known as inorganic benzene has 9 single bonds and 3 double bonds.
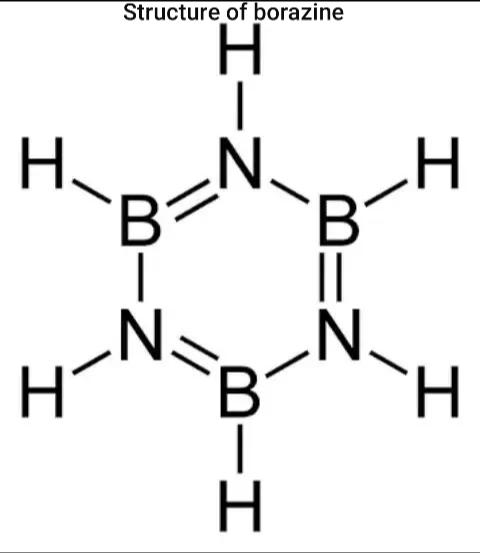
Hence, we get 12σ and 3πbonds and the correct option is option A.
Note: Borazole or borazine is iso-electronic and iso-structural with benzene and for this reason borazine is referred to as “inorganic benzene”. Though it is far more reactive than benzene owing to the electronegativity differences between Boron and Nitrogen.
