Question
Question: The bond present in\(\left( {{{\rm{N}}_{\rm{2}}}{{\rm{O}}_{\rm{5}}}} \right)\) are: A) Only ionic ...
The bond present in(N2O5) are:
A) Only ionic
B) Covalent
C) Dative
D) Both ionic and covalent
Solution
We know that the attracting between two atoms or molecules and the formation of new bonds in any chemical species defines the phenomena of chemical bonding. There are mainly two types of bond one is the ionic bond, and another one is the covalent bond.
Complete answer:
As we all know, when two non-metals bonded, they usually formed a covalent bond. In the N2O5 compound, there are two nitrogen atom and five oxygen atom. The name of given chemical formula N2O5 is dinitrogen pentoxide. It is one of the renowned oxides of the nitrogen atom. The atomic number of nitrogen is seven and oxygen is eight.
Generally, the valence electrons of nitrogen are three, and the valence electrons of oxygen are four. They both come under the category of non-metal. Thus, the bond present in the compound N2O5 is four N-O and two N=O. They all are covalent bonds because the sharing of electrons are shown by these atoms. So, the bond present in the N2O5 are covalent bond. Its structure is shown below.
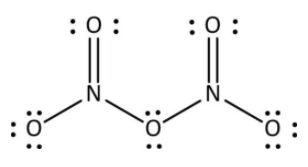
Hence, the correct answer is option ‘B’ i.e, a covalent bond.
Note: In the covalent bonds mainly sharing of electrons of any chemical species takes place. They are commonly termed as molecular bonds also. Electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.
