Question
Question: The bond order of \(C_{2}^{+}\) is (A) 1 (B) 2 (C) \(\dfrac{3}{2}\) (D) \(\dfrac{1}{2}\)...
The bond order of C2+ is
(A) 1
(B) 2
(C) 23
(D) 21
Solution
To solve this question, we first need to know what is bond order. The number of chemical bonds through which a pair of atoms are bonded is known as the bond order. The bond order of a molecule can be determined through the concept of molecular orbital theory.
Complete answer:
Now, to determine the bond order of C2+, we first need to draw its molecular orbital diagram.
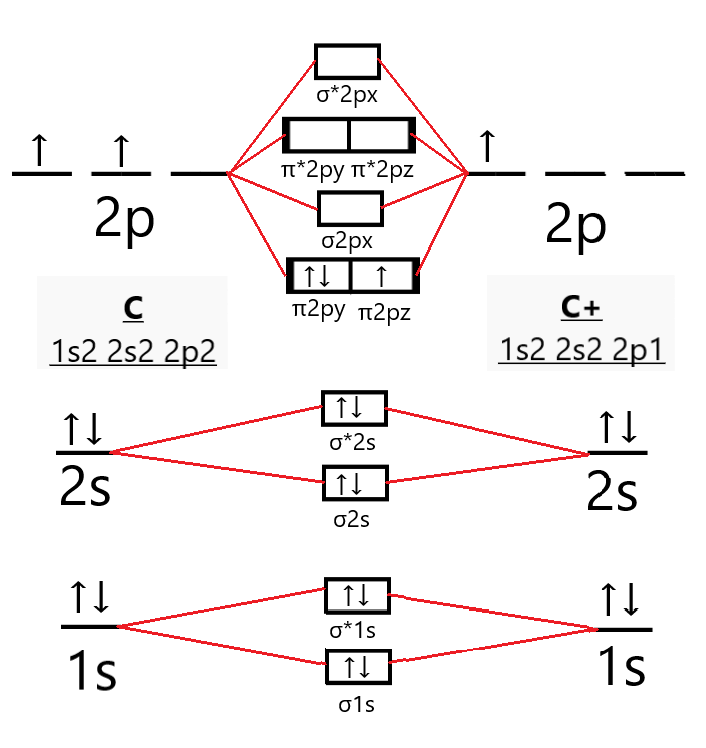
So, the electronic configuration of the C2+ molecule according to the molecular orbital theory is
C2+=(σ1s)2(σ∗1s)2(σ2s)2(σ∗2s)2(π2py)2(π2pz)1
Where σ/π orbitals depict bonding molecular orbitals whereas π∗/σ∗ depict antibonding molecular orbitals.
Now, the bond order of a molecule is given by the formula
BO=21[Nb−Na]
Where the number of electrons in the bonding orbitals is denoted by Nb and the number of electrons in the antibonding orbitals is denoted by Na.
From the molecular orbital diagram of the C2+ molecule we can see that it has 7 electrons in its bonding orbitals (Nb) and 4 electrons in its antibonding orbitals (Na).
So, its bond order will be
