Question
Question: The bond dissociation energy of B – F in \(B{{F}_{3}}\) is 646KJ/mol whereas that of C – F in \(C{{F...
The bond dissociation energy of B – F in BF3 is 646KJ/mol whereas that of C – F in CF4 is 515KJ/mol. Comment on the above statements.
[A] Stronger σ bond between B and F in BF3 as compared to that between C and F in CF4
[B] Significant pπ−pπ interaction between B and F in BF3 whereas no possibility of such interaction between C and F in CF4
[C] Lower degree of pπ−pπ interaction between B and F in BF3than that between C and F in CF4
[D] Smaller size of B atom as compared to C atom.
Solution
HINT: Bond dissociation energy is the energy required for the breaking of a bond. A sigma and a pi-bond required higher dissociation energy than a sigma-bond. See the bonding in the given compounds to answer the question.
COMPLETE STEP BY STEP SOLUTION: To solve this question, firstly we have to understand what bond dissociation energy is.
The energy required for the breaking of a bond is known as the bond dissociation energy. It gives us the strength of a chemical bond. Stronger the bond, higher is the energy required to break the bond and thus higher is the bond dissociation energy.
Now, in the question it is given to us that the bond dissociation energy of B – F in BF3 is 646KJ/mol whereas that of C – F in CF4 is 515KJ/mol so we can understand that the B – F bond is stronger than the C – F bond.
So, let us now discuss the reason for the higher bond dissociation energy of B – F bond.
In BF3, after bonding with the 3 fluorine atoms, boron still has empty p-orbitals which can be undergo a back bonding with the energetically available p-orbitals of fluorine whereas in CF4, all the p-orbitals of carbon are engaged in bonding with the fluorine atoms and there is no possibility of back-bonding. We can represent the back bonding of B – F as-
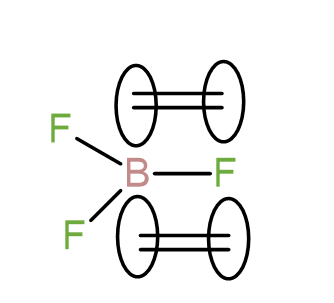
Due to this back bonding, the bond strength of B – F bond increases leading to increase in the bond dissociation energy.
We can understand from the above discussion that due to the presence of vacant p-orbitals boron can undergo back bonding with fluorine atoms leading to higher bond dissociation energy.
Therefore, the correct answer is option [B] Significant pπ−pπ interaction between B and F in BF3 whereas no possibility of such interaction between C and F in CF4.
NOTE: Bonds can be broken either homolytically or heterolytically. Bond dissociation energy usually refers to the homolytic case. Here, two electrons which were engaged in the formation of the bond go back to their original atoms as the bond breaks.
