Question
Question: The bond angle in \[\left( {{\rm{P}}{{\rm{H}}_{\rm{3}}}} \right)\] is A ) much lesser than \[{\rm...
The bond angle in (PH3) is
A ) much lesser than NH3
B ) equal to that in NH3
C ) much greater than in NH3
D ) slightly more than in NH3
Solution
Consider the type of orbitals (unhybridized or hybridised) that participate in each molecule. Phosphorus does not undergo hybridization while nitrogen does. Use this fact to estimate the bond angles.
Complete step by step answer:
In phosphene (PH3) molecule, the central phosphorus atom has one lone pair of electrons and three bond pairs of electrons. Phosphorus does not undergo hybridisation. Three phosphorus hydrogen bonds are formed by the overlap of three pure (unhybridized) p orbitals of phosphorus with 1s atomic orbitals of three hydrogen atoms. The lone pair of electrons is present in s orbital. The molecular geometry is trigonal pyramidal. The bond angle observed in phosphine is 93.5 degrees.
Phosphine is a Drago molecule. According to Drago’s rule, hybridisation will not take place if the central atom is in a third or higher period.
In ammonia (NH3) molecule, the central nitrogen atom has one lone pair of electrons and three bond pairs of electrons. Nitrogen undergoes sp3 hybridisation. The electron pair geometry is tetrahedral and the molecular geometry is trigonal pyramidal. The ideal bond angle in tetrahedral geometry is 108.28 degrees. However, the bond angle observed in ammonia is 107.48 degrees. Thus, the actual bond angle in ammonia is slightly lesser than the ideal tetrahedral bond angle. This is because, the lone pair - bond pair electron repulsions are greater than the bond pair - bond pair electron repulsions.
The structures of ammonia and phosphine are as shown below:
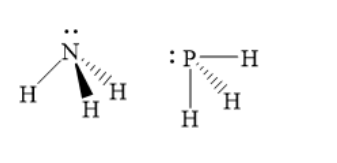
Thus, phosphine has much lower bond angle than ammonia. Hence, option A ) is the correct answer.
Note:
Do not say that both molecules undergo sp3 hybridisation and the bond angle in phosphine will be slightly more than that in ammonia as nitrogen is more electronegative than phosphorus.
