Question
Question: The boiling point of trans-dichloroethene is \[48^\circ C\]. Which number best approximates the boil...
The boiling point of trans-dichloroethene is 48∘C. Which number best approximates the boiling point of Cis-dichloroethene?
A.36 degree Celsius
B.42 degree Celsius
C.48 degree Celsius
D.54 degree Celsius
Solution
Isomerism is related to two or more compounds having the same molecular formula but a different arrangement of atoms within the molecule. Cis-trans isomerism is related to the position of the substituent groups on different sides of a double bond plane or a non-aromatic cycle. Cis-trans isomers are diastereomers. Diastereomers are isomers that do not mirror images of one another.
Complete step by step answer:
-The compounds having the same molecular formula but the different arrangements in molecular space are called isomers. Cis-trans isomerism is also a form of isomerism in which the compounds are differentiated based on the arrangement of the substituent groups on different sides of the double bond.
-The compound with the substituent groups on the same side of the double bond is considered Cis, while the compound where the groups are placed on either side of the double bond is considered Trans.
-The below image represents cis-dichloroethene and trans-dichloroethene respectively.
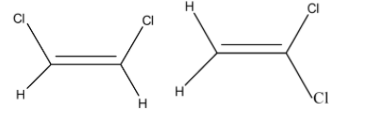
-The cis-trans isomers exist because of the high energy needed for the rotation around the double bond.
-Here the substituent group is chlorine which is a halogen.
-Due to higher molecular mass and great polarity the intermolecular forces of attraction (dipole-dipole and van der Waals) are stronger in halogen derivatives.
-Due to the placement of the halogens on either side of the bond the net dipole moment of the trans- isomer becomes 0, while in the case of cis-isomer the placement of the halogens on the same side of the bond increases the net dipole moment of the isomer.
-Since the dipole moment and resulting force of attraction is relatively weaker in the trans-isomer its boiling point is also less than its cis counterpart.
-The boiling point of compounds depends on the intermolecular forces operating between the molecules of the compound.
-Since the cis isomer has more effective intermolecular forces it tends to have a higher boiling point than its corresponding trans- isomer.
-The only option that has a more boiling point than the boiling point of trans-dichloroethene is D.
So the correct answer is option (D).
Note:
-Any of the six nonmetallic elements that constitute Group 17 of the periodic table are considered halogen.
-Halogens are usually electronegative and thus responsible for high intermolecular forces of attraction
-Two halogens on opposite sides of the double bond in trans-dichloroethene cancel the effect of each other.
-Two halogens on the same side of the double bond of cis-dichloroethene further strengthen the effect of each other.
