Question
Question: The binding energy per nucleon of iron atom is approximately- (A) \(13.6eV\) (B) \(8.8MeV\) (C...
The binding energy per nucleon of iron atom is approximately-
(A) 13.6eV
(B) 8.8MeV
(C) infinity
(D) 10MeV
Solution
Hint
To understand the solution to this problem we need to go through the binding energy versus mass number curve for the elements. From there we can see that the peak of the curve, corresponding to the most stable element, has a mass number of 56 which is iron and we can find the corresponding binding energy from the graph.
Complete Step by Step Solution
For a nucleus, the binding energy is the minimum energy which is necessary to separate out the nucleons. When we add the total mass of all the nucleons, then it is always less than the mass of the nucleus. This disappeared mass gets converted into energy called the binding energy. To solve this problem we need to carefully understand the binding energy versus mass number curve as given in the figure below.
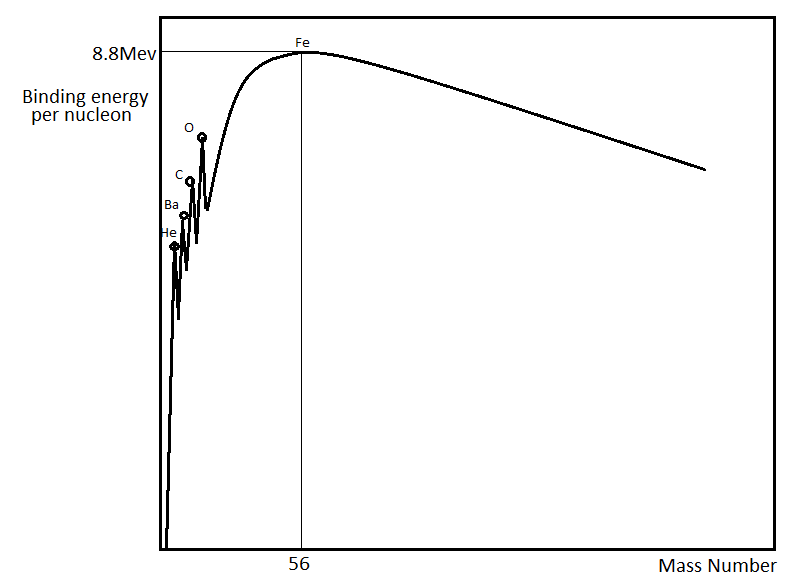
From the figure, we can see that the binding energy per nucleon of the atoms increases rapidly as the mass number increases until it reaches a peak and then starts to gradually decrease.
The peak in the curve corresponds to the most stable nucleus, which corresponds to the mass number of 56. The mass number 56 belongs to the iron nucleusFe56. So from the curve, we can see that the binding energy per nucleon of the iron atom is around 8.8MeV and also that it is the most stable nucleus that exists in nature.
Hence, option B is correct.
Additional Information
From the curve, anything on the left side of Fe56 can be created in a fusion process, in which two small nuclei are compressed together in extreme temperature and pressure to form another element. And anything to the right of Fe56 can be created in the fission process, in which a heavy atom splits into lighter atoms.
Note
The iron atom having a binding energy of 8.8MeV, makes it one of the most tightly bound nuclei. The Fe56 is the most common isotope of iron and consists of about 91.75% of all iron. In the curve, we can see a few other peaks. These correspond to the nucleus of the elements He, Ba, C, and O. These nuclei are lying on peaks because they have a binding energy per nucleon relatively greater than that of the surrounding nuclei.
