Question
Question: The binding energies of two nuclei P and Q all and y joules. If 2x > y then the energy released in r...
The binding energies of two nuclei P and Q all and y joules. If 2x > y then the energy released in reaction
Pn+Pn→Q2n, will be
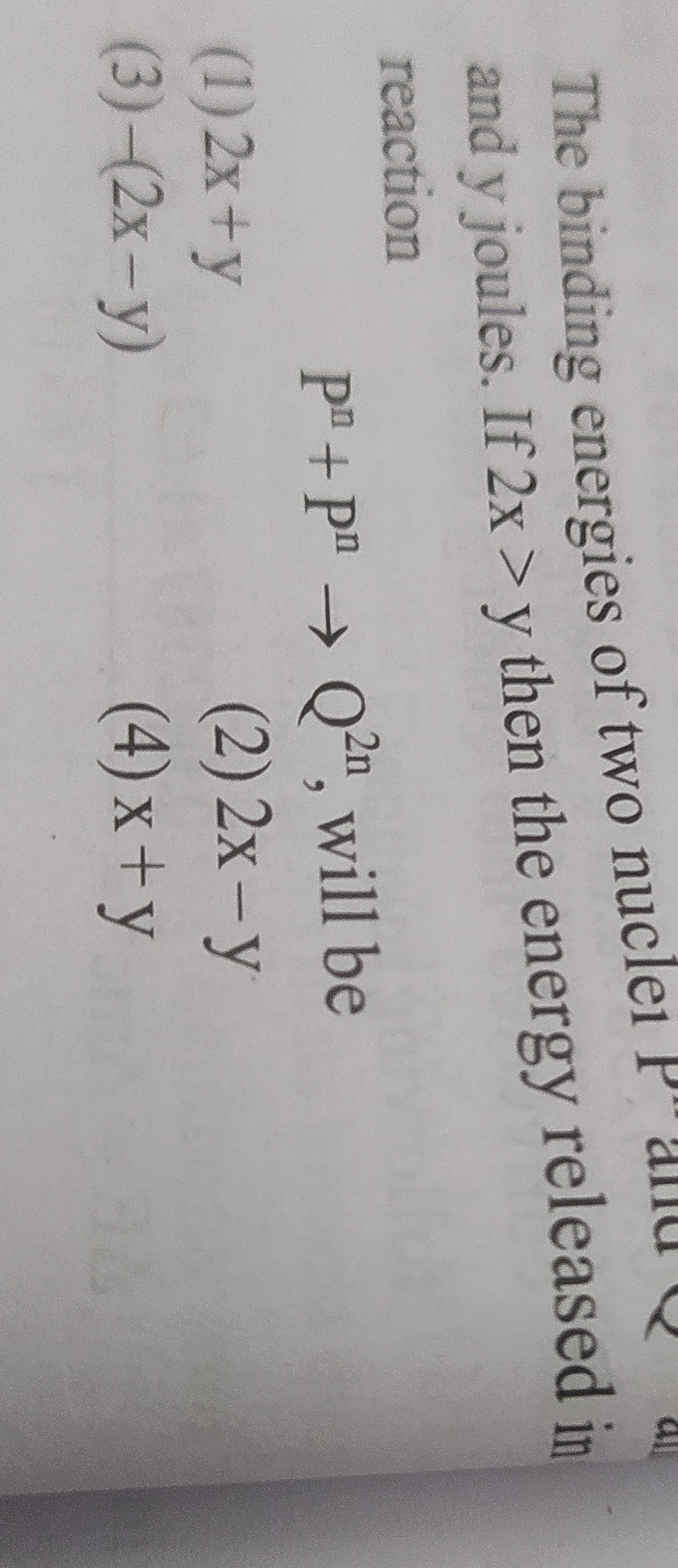
2x + y
2x - y
-(2x - y)
x + y
2x - y
Solution
The energy released in a nuclear reaction (Q-value) is given by the difference between the total binding energy of the products and the total binding energy of the reactants.
Given: Binding energy of nucleus P = x joules Binding energy of nucleus Q = y joules
The reaction is: Pn+Pn→Q2n
Total binding energy of reactants = Binding energy of P + Binding energy of P = x + x = 2x Total binding energy of products = Binding energy of Q = y
Energy released (Q) = (Total binding energy of products) - (Total binding energy of reactants) Q=y−2x
The problem states that 2x>y. This implies that y−2x will be a negative value.
However, in the context of multiple-choice questions asking for "energy released", if the calculated Q-value (BE_products - BE_reactants) is negative, it often implies that the question is asking for the magnitude of the energy change.
If the energy released is denoted by Q, and Q=y−2x, then since y−2x<0, the reaction absorbs energy. The amount of energy absorbed is −(y−2x)=2x−y.
Thus, the answer would be ∣y−2x∣. Since y−2x is negative, ∣y−2x∣=−(y−2x)=2x−y.
