Question
Question: The best method to separate the mixture of ortho and para-nitrophenol (1:1) is A.Vaporization ...
The best method to separate the mixture of ortho and para-nitrophenol (1:1) is
A.Vaporization
B.colour spectrum
C.distillation
D.crystallization
Solution
Draw the structures of ortho and para- nitrophenol. Determine the type of intermolecular forces present in both the compounds. Based on the intermolecular forces and boiling point determine the method of separation of a mixture of ortho and para-nitrophenol (1:1).
Step by step answer: The structure of ortho-nitrophenol is as follows:
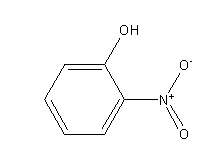
Ortho-nitrophenol
The structure of para-nitrophenol is as follows:
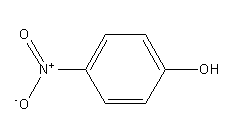
Para-nitrophenol
Determine the type of intermolecular forces present in ortho and para- nitrophenol.
In the case of ortho-nitrophenol since hydroxyl and nitro groups are adjacent to each other so it shows intramolecular hydrogen bonding.
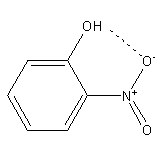
Intramolecular hydrogen bonding
In the case of para-nitrophenol since hydroxyl and nitro groups are opposite to each other so it shows intermolecular hydrogen bonding.
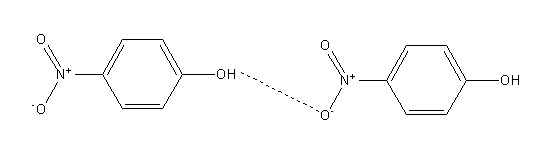
Intermolecular hydrogen bonding
As intermolecular hydrogen bonding is stronger than the intramolecular hydrogen bonding so para-nitrophenol has a higher boiling point than the ortho-nitrophenol. The distillation method is used to separate the mixture of liquid having a considerable difference in boiling point. In the distillation method liquid having a lower boiling point converted into vapor first and separated from the mixture of liquid. In this case, since ortho-nitrophenol has a lower boiling point than para-nitrophenol so from the mixture ortho-nitrophenol will boil at a lower temperature and be separated first from the mixture.
Thus, the correct option is (C) distillation.
Note: There are various methods of separation depending on the type of mixture. To separate the liquid-liquid mixture having different boiling points the method used is distillation. The boiling point of a liquid depends on the type of intermolecular forces. The stronger the intermolecular forces are, the greater is the boiling point.
