Question
Question: The best indicator for the detection of the end point in the titration of a weak acid and a strong b...
The best indicator for the detection of the end point in the titration of a weak acid and a strong base is:
A. Methyl orange ( pH range 3 to 4 )
B. Methyl red ( pH range 4 to 6 )
C. Thymol blue ( pH range 8 to 3 )
D. Phenolphthalein ( pH range 8 to 10 )
Solution
The best indicator used in the detection of the end point in the titration of a weak and a strong base is the chemical compound with formula C20H14O8 . It turns colorless when applied in acidic solution and pink when applied in basic solution.
Complete answer:
Phenolphthalein is a compound with formula C20H14O8 . Its IUPAC name is 3,3−Bis(4−hydroxyphenyl)−2−benzofuran−1(3H)−one . It appeared as a white powder. It is used in acid-base titration. In the case of acidic solution the end point is detected if the solution turns colorless. And in the case of a basic solution the endpoint is detected if the solution gets turned into a pink color solution.
If the case of titrating weak acid and strong base, phenolphthalein convert the solution color to pink which indicate the end point of the solution.
Phenolphthalein shows four different colors for detecting an aqueous solution depending on the pH of the solution. As if the solution is strongly acidic then it exists in protonated form ( Hln+ ), and gives an orange coloration. if the solution is in between strongly acidic and slightly basic conditions, the lactone form is a colorless solution. The doubly deprotonated ( ln2− ) phenolate form which gives the pink color to the solution. If the solution is strongly basic in nature then phenolphthalein is converted to its ln(OH)3− form, and then its pink color changes and fades which becomes completely colorless above 13.0pH .
Structure of phenolphthalein is gives as:
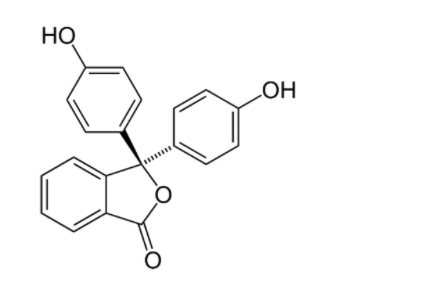
Hence, option D is correct.
Note:
Phenolphthalein is a weak acid. It can lose H+ ions in solution. The non-ionized phenolphthalein molecule is colorless and the protonated phenolphthalein ion is an orange. The deprotonated phenolphthalein ion is fuchsia. Sensitivity of phenolphthalein is used in other applications such as concrete that has naturally high pH due to the calcium hydroxide formed when Portland cement reacts with water. As the concrete reacts with carbon dioxide in the atmosphere, pH decreases to 8.5−9 .
