Question
Question: The \[BC{{l}_{3}}\] is planar molecule, whereas \[NC{{l}_{3}}\] is a pyramidal because: a.) N — Cl...
The BCl3 is planar molecule, whereas NCl3 is a pyramidal because:
a.) N — Cl bond more covalent bond than B — Cl bond.
b.) B — Cl bond is more polar than N — Cl bond.
c.) nitrogen atom is similar to boron atom.
d.) BCl3 has no lone pair but NCl3 has a lone pair or electrons.
Solution
Hint: Molecular geometry is the three-dimensional arrangement of a molecule constituting the general shape as well as the bond length, bond angle and other geometrical parameters that is useful for determining the position of each atom.
Complete step by step solution:
According to the valence shell electron pair repulsion theory (VSEPR), molecules try to attain a geometry that minimizes the repulsion between electrons in the valence shell of the atom.
The valence shell is taken as a sphere and with electrons localizing on the spherical surface at maximum distance from one another.
Now given in the question BCl3 is a planar molecule, we know that boron has 3 valence electrons, it will form a covalent bond with each of the chlorine atoms. Therefore, 3 bond pairs will be formed. There will be no lone pairs as all the valence electrons of boron will make a bond with the 3 chlorine atoms.
Now the molecule will try to attain a geometry that minimizes the repulsion between the bond pair - bond pair, forming an angle of 120 degree between all the bonds.
On arranging all the bond pairs BCl3 forms a trigonal planar shape. 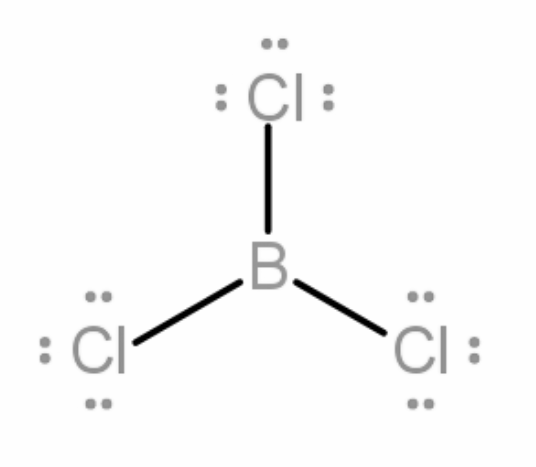
Similarly, in NCl3, Nitrogen has 5 valence electrons, it will form a covalent bond with each of the chlorine atoms. Therefore, 3 bond pairs will be formed. There will be one lone pair left as only 3 of the 5 valence electrons are used in bond formation.
Now the molecule will try to attain a geometry that minimizes the repulsion between the bond pair - lone pair, forming an angle of 109.5 degree between all the bonds.
On arranging all the bond pairs NCl3 forms a pyramidal shape. 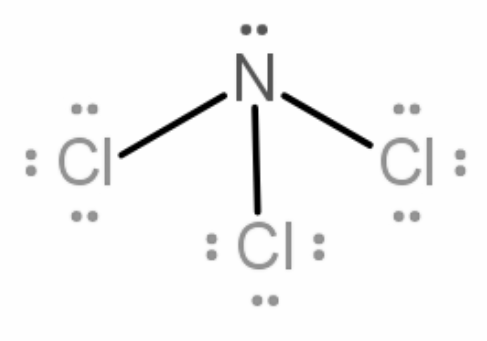
Therefore, we can conclude that the BCl3 is planar because it has no lone pairs and NCl3 is pyramidal as it has one lone pair of electrons. So, the correct option is (d).
Note: Geometry of a molecule is the arrangement of (lone pair + bond pair) around the central atom and it corresponds to the coordination number of the molecule while shape is the structure of the molecule excluding the lone pair on the central atom. Lone pairs are not considered when we determine the shape of the molecules.
