Question
Question: The B-F bond length in \(M{{e}_{3}}N.B{{F}_{3}}\) is 1.35 A. much longer than 1.30 A in \(B{{F}_{3}}...
The B-F bond length in Me3N.BF3 is 1.35 A. much longer than 1.30 A in BF3 . Explain
Solution
Complete step by step answer:
- In the question it is given that the B-F bond length in Me3N.BF3 is 1.35 A but the B-F bond length is 1.30 A.
- We have to find the reason behind the high B-F bond length in Me3N.BF3 .
- We know that there is a double bond character in between boron and fluorine in boron trifluoride.
- When boron trifluoride reacts with tri methyl amine we can observe the formation of adduct Me3N.BF3 .
- Because of this adduct formation there is a new bond that is going to form between nitrogen and boron in boron trifluoride.
- Then there is no chance of the presence of the back bonding in boron trifluoride.
- That is why the B-F bond length is high in Me3N.BF3 is 1.35 A (high) when compared to 1.35 A in boron trifluoride.
- We can see the formed adduct ( Me3N.BF3) when boron trifluoride reacts with tri methyl amine is as follows.
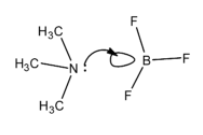
Note: The length of the single bond is higher when compared to double bond. Therefore the length of the B-F bond (1.30 A) is less in boron trifluoride when compared to B-F bond length (1.35 A) in Me3N.BF3.
