Question
Question: The average oxidation state of chlorine in bleaching powder is: A. -1 B. +1 C. Zero D. -2 as...
The average oxidation state of chlorine in bleaching powder is:
A. -1
B. +1
C. Zero
D. -2 as well as +2
Solution
We know that the molecular formula of bleaching powder is Ca(OCl)Cl. In the molecular structure of bleaching powder, Cais the centred atom with OCl and Cl ions being branched to it.
Complete answer:
The structure of Ca2+(OCl)−Cl− is as follows:
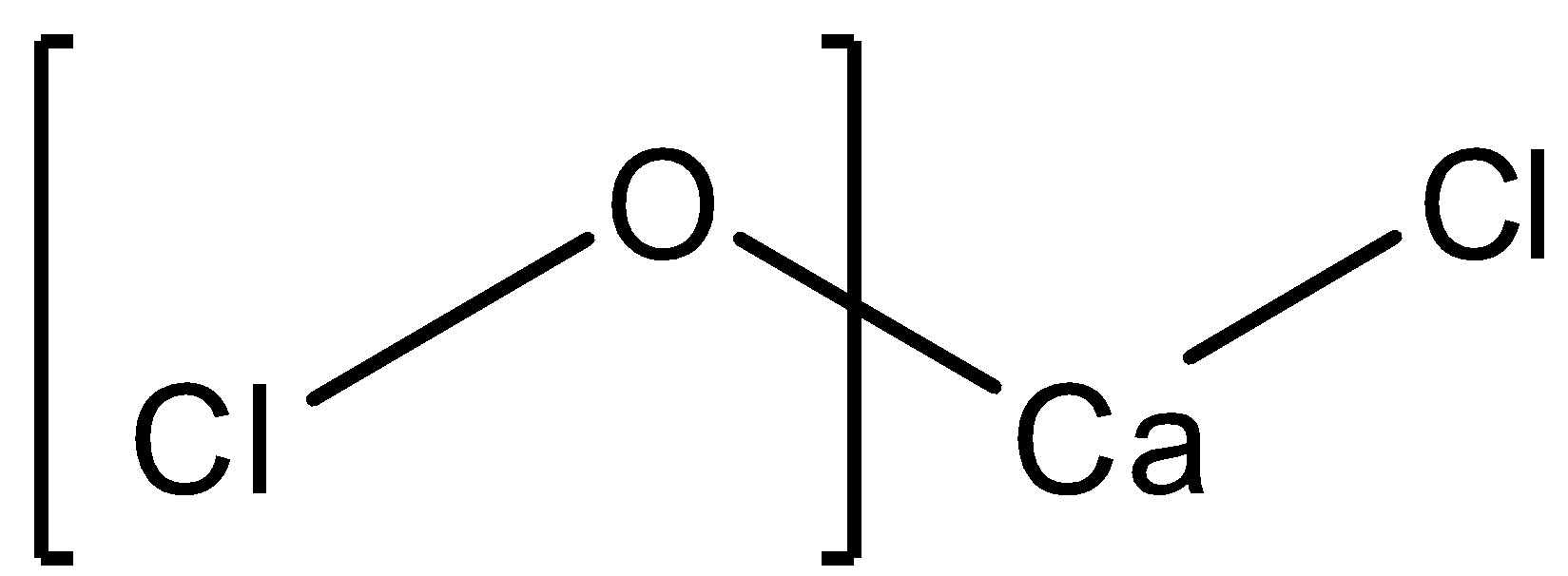
We can see that calcium having a valency of +2, gains electrons from chlorine and oxygen. While oxygen having a valency of -2 shares an electron with the other chlorine. This proves that the chlorine atom attached directly to calcium has an oxidation state of -1. Calcium itself has an oxidation state of +2 which brings the following equation stating the oxidation state of OCl to be -1. The sum of the oxidation states of all the atoms is 0 since the molecule is neutral.
+2 + (−1) + (−1) = 0Ca Cl OCl Stability
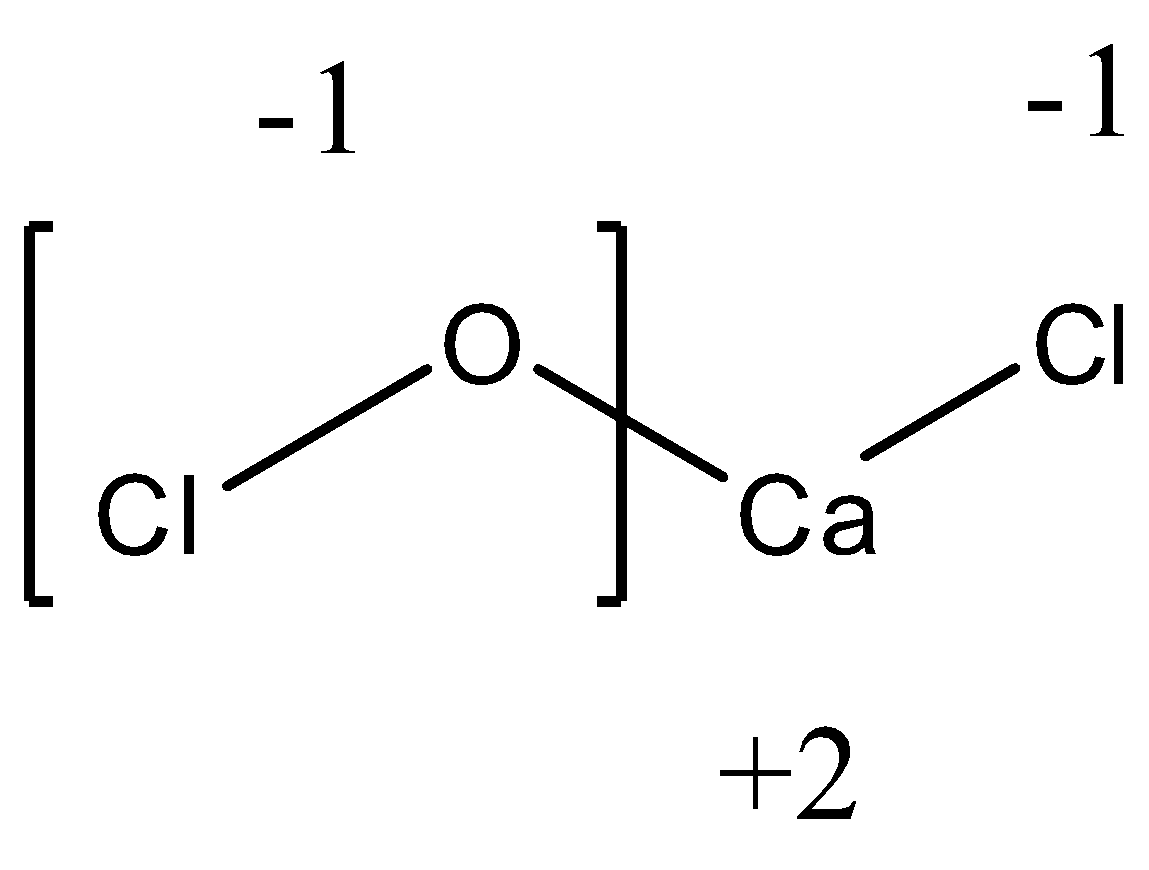
In the above equation, we see that stability is reached by the Ca(OCl)Cl molecule when the oxidation state of OCl is -1. We know that the oxidation state of oxygen is -2. Hence, to bring the oxidation state of OCl as -1, the Chlorine atom attached to Oxygen must have an oxidation state of +1 as calculated from the following equation.
(−2) + x=(−1)
Where, x is the oxidation state of chlorine atom attached to oxygen.
From the above equation, we get the oxidation state of chlorine attached to oxygen to be +1.
Therefore, oxidation states of Cl=+1,−1
Hence, average oxidation state ofCl= 2(+1)+(−1) = 20 = 0
The average oxidation state of Cl atom in Ca(OCl)Cl is zero.
Therefore, the correct answer is Option C.
Note: We should be knowing that bleaching powder is yellow in colour, having a strong smell of chlorine. It is soluble in water but aqueous solution is never found due to the presence of impurities. It loses chlorine by the action of carbon dioxide.
Although the usual oxidation state of chlorine is -1, since oxygen is more electronegative than chlorine, it adapts to the +1 oxidation state.
