Question
Question: The average kinetic energy of the particles of a gas is most closely associated with which of the fo...
The average kinetic energy of the particles of a gas is most closely associated with which of the following quantity?
A) Heat capacity
B) temperature
C) specific heat
D) absolute zero
E) potential energy
Solution
The particles of a gas are supposed to have elastic collisions both with the wall of the container and the other particles ideally. They are thought of as point mass particles with a velocity range zero to infinity. Hence, their average kinetic energy is to be calculated taking care of this assumptions.
Complete step by step answer:
Step 1:
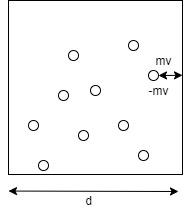
Let in an one dimensional case, a gas particle with mass m collides with the wall of a container with length d perpendicularly with a velocity v and exerts a force F as
You can find the frequency of the collision as
t1=dv
So, the average force becomes
F=dmv2
Step 2:
Calculate the total force on the wall due to all the particles
F=∑dmv2 =LmN[N∑v2] =LmNvrms2
Step 3:
You can exploit the symmetry, hence for a three-dimensional case
vxyz2=vx2+vy2+vz2=3v2
Hence, you get the force F as
F=3dmNvrms2
Calculate the pressure on the wall of the container as
P=3d3mNvrms2
Now the volume of the container is the total volume of the gas particles as
V=d3
Hence you can have
Step 4:
The average kinetic energy of a gas particle is
K=21mvrms2
Hence, you can rewrite the relation in eq (1) as
PV=32N×21mvrms2 =32NK
Step 5:
Now from ideal gas relation you have
PV=NkbT
where, kb is the universal Boltzmann constant and T is the temperature of the gas.
Calculate the average translational kinetic energy of a gas particle by using the relation in eq (2)
32NK=NkbT ⇒K=23kbT
Hence, you can see that the average kinetic energy can be solely determined from the temperature T and the universal Boltzmann constant kb .
Final Answer:
The average kinetic energy of the particles of a gas is most closely associated with (B) temperature.
Note: The assumption of elastic collision is to be carefully taken in case of calculation of change of the momentum. The pressure should be calculated on the wall of the container due to the force exerted by the particles. The collisional effects between the molecules are not considered significant as they can be average out to zero in the long run. The other quantities like heat capacity and specific heat can affect the average kinetic energy but not a bit as significant as the temperature as you saw from the derivation.
