Question
Question: The average charge on each o atom and average bond order of I-O bond in \(\text{I}{{\text{O}}_{6}}^{...
The average charge on each o atom and average bond order of I-O bond in IO65− is
(A) −1 and 1⋅67
(B) −5/6 and 1⋅33
(C) −5/6 and 1⋅67
(D) −5/6 and 1⋅167
Solution
Hint IO65−: I is central atom where I surrounds by the six oxygen atom with negative charge. In this structure we have to calculate average charge and average bond order.
Average charge=No. of atom responsible for chargeTotal charge of molecule
Bond order=Total atom surroundingTotal bonds
Complete step by step solution:
InIO65− the structure as follows
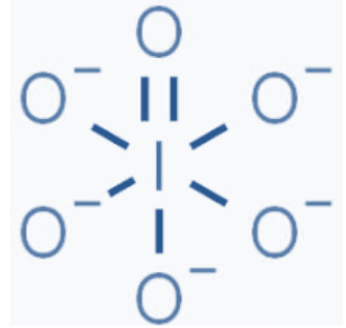
Charge on each oxygen atom=1
Total charge on molecule contributed by oxygen atom=−5
Average charge=No. of total oxygen atomCharge on molecule
⟹6−5
Average bond order:
No. of total bonds=7 [as one is double bond and 5 is single bond]
Total no. of oxygen
⟹67⟹1⋅16
So the correct option is ‘D’
Note: Covalent bond: A bond which is formed by the shared pair of electrons is called covalent bond. Further covalent bond divided into two kinds.
Sigma Bond: A bond which is formed by overlapping of the internuclear axis is called sigma bond. It is of three kinds S-S overlapping, S-P overlapping, P-P overlapping.
Pi-Bond: A bond which is formed by the sideways overlapping of P-orbital is called pi-bond
