Question
Question: The average atomic mass of a mixture containing 79 mole % of $^{24}$Mg and remaining 21 mole % of $^...
The average atomic mass of a mixture containing 79 mole % of 24Mg and remaining 21 mole % of 25Mg and 26Mg, is 24.31. % mole of 26Mg is
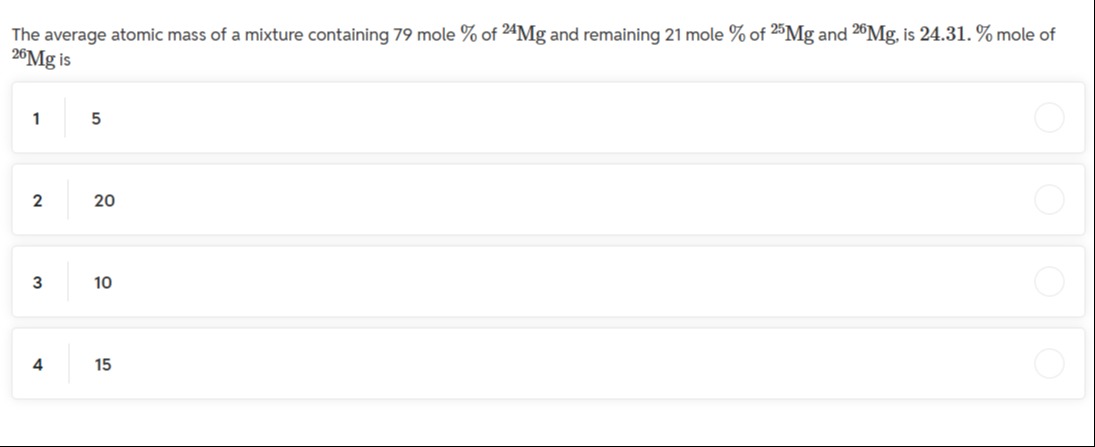
A
5
B
20
C
10
D
15
Answer
10
Explanation
Solution
The average atomic mass of an element is calculated as the weighted average of the masses of its isotopes.
Given:
- Average atomic mass of Mg = 24.31
- Mole % of 24Mg = 79%
- Remaining 21 mole % is a mixture of 25Mg and 26Mg.
Let the mole % of 26Mg be x. Then, the mole % of 25Mg will be (21−x).
The approximate atomic masses of the isotopes are:
- 24Mg: 24 amu
- 25Mg: 25 amu
- 26Mg: 26 amu
Using the formula for average atomic mass:
Average Atomic Mass=∑(Isotopic Mass×Abundance) 24.31=(24×10079)+(25×10021−x)+(26×100x)Multiply the entire equation by 100:
24.31×100=(24×79)+(25×(21−x))+(26×x) 2431=1896+(525−25x)+26xCombine the constant terms and the terms with x:
2431=(1896+525)+(−25x+26x) 2431=2421+xNow, solve for x:
x=2431−2421 x=10Therefore, the mole % of 26Mg is 10%.
