Question
Question: The atoms of different elements having the same number of neutrons is known as: A.Isobars B.Isom...
The atoms of different elements having the same number of neutrons is known as:
A.Isobars
B.Isomers
C.Isotones
D.Isotopes
Solution
When encountering such terms like ‘isobars’, we must understand what they mean. The word ‘iso’ stands for the same. To solve this question, we will first understand the definition of each term mentioned and then interpret the correct option.
Complete step by step answer:
Isotopes are defined as the group of the same elements that have different numbers of neutrons but the same number of protons and electrons.
Example – The isotopes of Hydrogen are – 11H - Protium, 12H - Deuterium and 13H - Tritium.
Isomers are molecules that have the same molecular formula but different structural formula.
Example – The isomers of butane are –
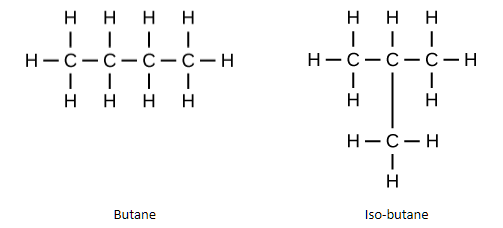
Isotones are a group of atoms of different elements that have the same number of neutrons but different number of protons.
Example – Oxygen 816O , Nitrogen 715N and Carbon 614C have same number of neutrons, i.e., 8. Hence, they are called isotones.
Isobars are different elements that have different atomic numbers but have the same mass number.
Example – An example of pair of isobars is 40S,40Ca,40K,40Ar,40Cl .
Hence, the correct answer is (C) i.e., isotones.
Note: In a given pair of isotones, the element with the higher atomic number, would have the higher atomic mass number out of the two. This tells us that the mass of the element with the higher atomic number would be higher. So, this gives us more information about isotones.
