Question
Question: The atomic number of the central atom in \(MC{{l}_{2}}\)is 50. The shape of gaseous \(MC{{l}_{2}}\) ...
The atomic number of the central atom in MCl2is 50. The shape of gaseous MCl2 is given as:
A.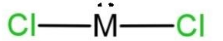
B.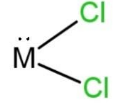
C.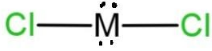
D.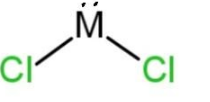
Solution
Shape of any molecule depends upon the bonded pairs of electrons, as well as the non-bonded, free electrons. Shapes of various molecules are derived through VSEPR theory.
Complete step by step solution:
Given is the molecule with formula MCl2, the molecule has atomic number 50. So, the 2 chlorine atoms will have Cl2 = 17 + 17 = 34,
Therefore, 34 – 50 = 16, which is the atomic number of the central atom. This means that the central atom is sulfur, S. So the molecule will have two lone pairs and 2 bond pairs of electrons. As the total valence electrons will be 14, 6 from which placed on both the chlorine atoms, remaining 2 lone pairs placed on the sulfur, here M central atom. So, the shape of this molecule will be bent due to the presence of 2 lone pairs and 2 bond pairs. The shape with 2 lone pairs is C, which is
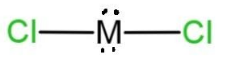
Hence, option C is correct, but the shape is bent.
Note: The shape of molecules is determined by the VSEPR theory, that states that lone pairs are localized on a central atom and occupy more space in comparison with bond pairs, which distorts the shape of the molecule. Hence the two lone pairs are placed on the central atom that changes its shape from linear to bent. This is an example of irregular geometry in VSEPR theory.
