Question
Question: The \[As{F_5}\] molecule is trigonal bipyramidal. The hybrid orbitals used by the atoms for bonding ...
The AsF5 molecule is trigonal bipyramidal. The hybrid orbitals used by the atoms for bonding are:
(A) dx2−y2,dz2,px,py
(B) dxy,s,px,py,pz
(C) dx2−y2,s,px,py
(D) s,px,py,pz,dz2
Solution
Ground state electronic configuration of Arsenic atom is [Ar]3d104s24p3. dx2−y2 orbital is situated in the xy plane and dz2 is situated at the z-axis and perpendicular to xy plane.
Complete answer:
We know that Arsenic is the element of the Nitrogen group. So, its ground state electronic configuration is [Ar]3d104s24p3 and excited state configuration will be [Ar]3d104s14p34d1.
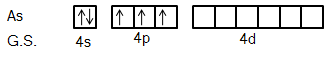
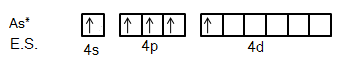
So, we can say from this excited state of Arsenic atom that it can bind with five Fluorine atoms with five different orbitals.
It is clear from the figure that five fluorine atoms will give one electron each to five half-filled orbitals of Arsenic atoms. So, hybridization in this compound will be sp3d.
So, it is evident that s,px,py,pz orbitals will take part because they are only available orbitals.
Now, if sp3d hybridized compounds have trigonal bipyramidal geometry, then it has to form bonds in a plane and two more bonds perpendicular to the plane. If we take the plane in xy direction, then only one orbital is there which can form bonds perpendicular to it and it is dz2 as it is along the z-axis.
So, orbitals involved are s,px,py,pz,dz2
So, the correct answer is (D) s,px,py,pz,dz2.
Additional Information:
- In sp3d hybridization, compound can have either trigonal bipyramidal or square pyramidal geometry.
- If a compound is having trigonal bipyramidal geometry, then it uses dz2 orbital as one of the d-orbitals to form bonds. If a compound has square pyramidal geometry, then dx2−y2 is used as one of the d-orbitals to make bonds.
Note: Do not consider that more than one d-orbitals take part in the hybridization as one s-orbital and 3 p-orbitals are already taking part in the hybridization. Make sure that you decide hybridization from the excited state of the atom and not the ground state.
