Question
Question: The anode voltage of a photocell is kept fixed. The wavelength \(\lambda\) of the light falling on t...
The anode voltage of a photocell is kept fixed. The wavelength λ of the light falling on the cathode is gradually changed. The plate current I of the photocell varies as follows:
A.
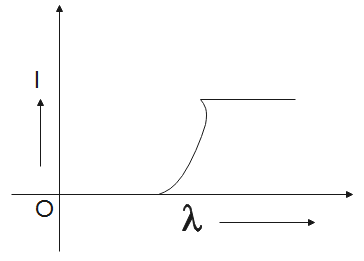
B.
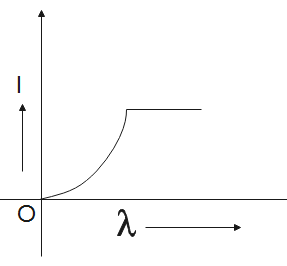
C.
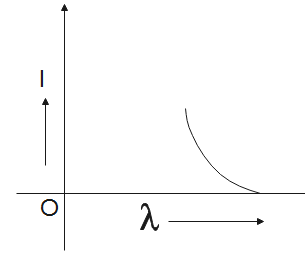
D.
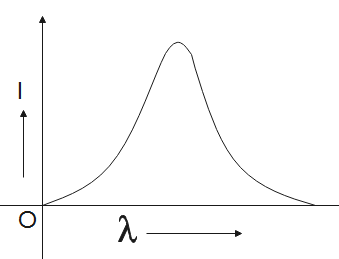
Solution
Use concept of the photoelectric effect. Find the relation between the maximum possible kinetic energy of the photoelectrons and the wavelength of the light incident on the metal plate. If maximum possible kinetic energy of the electrons increases, more of the number of electrons will reach the anode terminal. Hence, the current will increase. Then select the graph will suit best for the concluded data.
Formula used:
E=λhc
Kmax=λhc−ϕ
Complete step-by-step answer:
When a light with suitable wavelength falls on a metal plate, electrons are ejected out of the surface of the metal. Light is made up of massless particles called photons. Energy of a photon is equal to E=λhc, where h is the Planck’s constant and c is the speed of light in vacuum.
When light is incident on the metal, each photon collides with one electron and transfers its energy to the electron. If the energy of the photon is greater than the work function (ϕ) of the metal, then the electron will be ejected out of the metal with some kinetic energy. And the ejected electron (called photoelectron) travels from the cathode to anode which leads to a current in the circuit.
Work function is the minimum energy needed to remove the electron from the metal.
The maximum possible kinetic energy of the photoelectrons is given as Kmax=λhc−ϕ.
The work function for a given metal is constant. h and c are also constants. Therefore, from (i) we get that the maximum kinetic energy of the electrons is inversely proportional to the wavelength of light.
If maximum possible kinetic energy of the electrons increases, more of the number of electrons will reach the anode terminal. Hence, the current will increase.
For the value of Kmax to increase, the value ofλ must decrease. Hence, as the wavelength of the light reduces the current increases.
As the wavelength increases, the current decreases. There will be a maximum value of the wavelength where the energy of the photons will be equal to the work function of the metal. If the wavelength is increased beyond this value, the energy of the photons will be less than the work function and no electrons will be ejected. Hence, there will be no current beyond the maximum value of the wavelength.
Now, the graph will be the most suitable for the concluded data is graph C.
So, the correct answer is “Option C”.
Note: The current in the circuit also depends on the intensity of the light. Intensity of the light is directly proportional to the number of photons. We know that each photon produced one photoelectron. Therefore, if the number of photons increases, then the number of photoelectrons will increase. Thus, the current in the circuit will increase.
And for more number of the photons, the intensity of the light must be greater. Hence, the current in the circuit increases with increase in the intensity of the light.
