Question
Question: The analgesic drug ibuprofen (A) is chiral and exists in (+) and (-) forms. One enantiomer is physio...
The analgesic drug ibuprofen (A) is chiral and exists in (+) and (-) forms. One enantiomer is physiologically active, while the other is inactive. The structure of ibuprofen is given below:
The IUPAC name of (A) is:
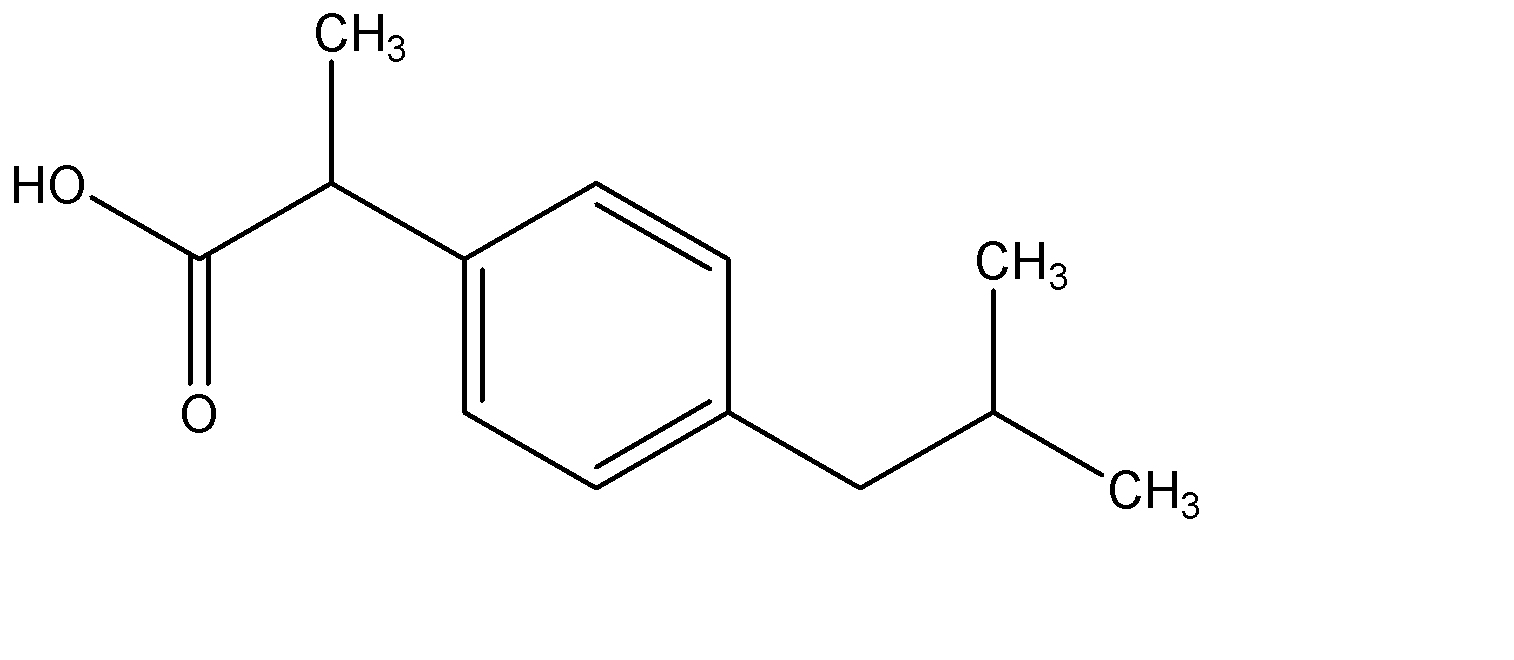
A. 3 - (p-isobutyl phenyl) propanoic acid
B. 2 - (p-isobutyl phenyl) propanoic acid
C. 3 - (p-sec-butyl phenyl) propanoic acid
D.2 - (p-sec-butyl phenyl) propanoic acid
Solution
To solve this question, we must first draw the molecular structure of Ibuprofen. Then on the basis of the IUPAC rules, we can name the given compound.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
The nomenclature rules of IUPAC can be given as:
1.Find the longest chain of carbon atoms. This will be the parent chain of the compound.
2.Then identify all the substituents of the functional groups.
3.While numbering the functional group, the numbering on the parent chain should be such that it should be closest to the terminal carbon atom.
4.If the same functional group is occurring more than once, then while mentioning the functional group in the name of the hydrocarbon, appropriate position numbering should be mentioned. In addition, the number of times the substituent group occurs is indicated by a prefix (di, tri, tetra, etc.).
5.If chains of equal length are competing for selection as the parent chain, then the choice goes in series to:
a) the chain which has the greatest number of side chains.
b) the chain whose substituents have the lowest- numbers.
c) the chain having the greatest number of carbon atoms in the smaller side chain.
d)the chain having the least branched side chains.
Hence, we can see that the parent chain or the longest chain in the compound is the one with 3 carbon atoms. Hence propane is the parent chain. Now, the terminal carbon of this propane has a carboxylic functional group. Hence the parent chain is now propanoic acid.
On the second carbon atom in the chain, we have a few substituents attached. Let us name them:
First, we see a phenyl group, which is linked to the second carbon of the present chain at position 1. At position 4 of the phenyl group, we have the functional group named 2 – methyl butane, which is commonly known as isobutane. Since this chain is attached at position 4 of phenyl, it is at para position. Hence the name of the entire substituent can be given as p – isobutyl phenyl. And since this is attached at the second carbon atom of the parent chain, the complete name of this compound is given as:
2 - (p-isobutyl phenyl) propanoic acid
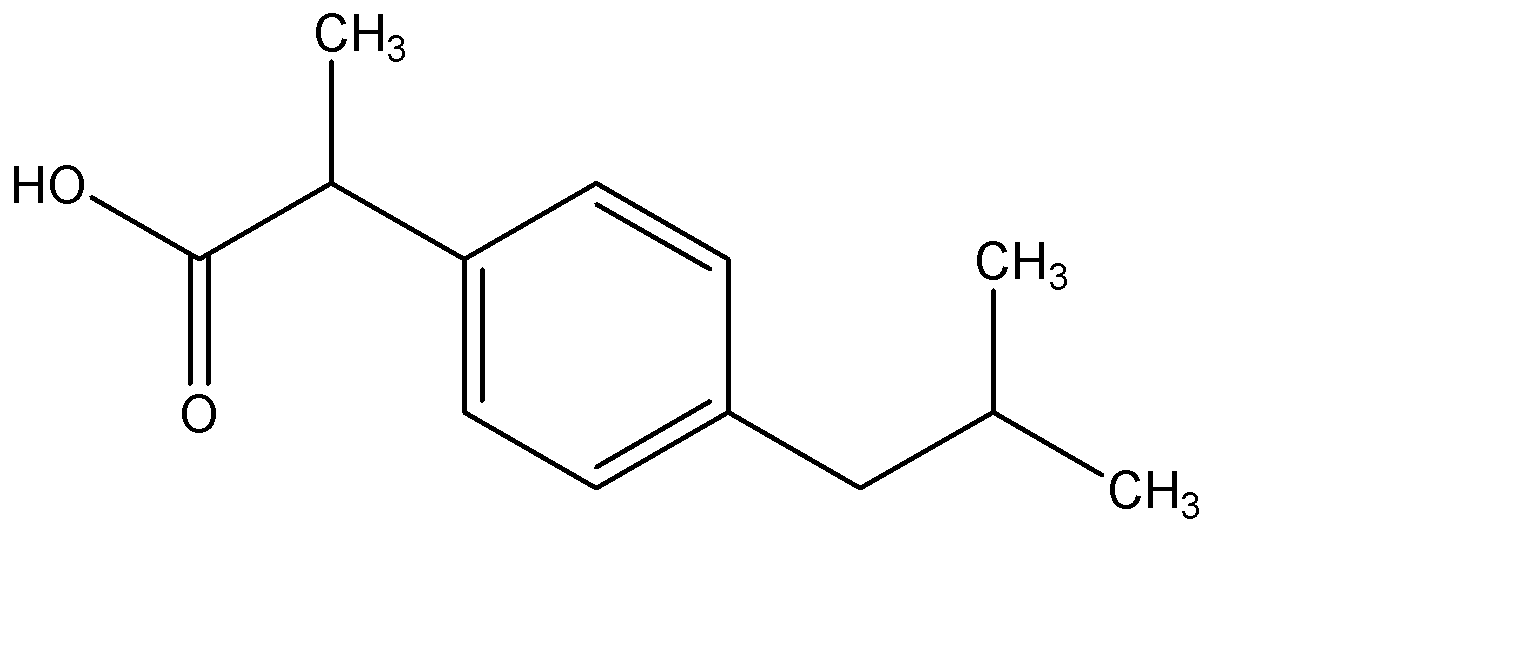
Hence, Option B is the correct option
Note: Non-prescription ibuprofen is used to reduce fever and to relieve minor aches and pain from headaches, muscle aches, arthritis, menstrual periods, the common cold, toothaches, and backaches. Ibuprofen is in a class of medications called NSAIDs.
