Question
Question: The alkene that exhibits geometrical isomerism is: A. 2- methylpropene B. Propene C. But-2-ene...
The alkene that exhibits geometrical isomerism is:
A. 2- methylpropene
B. Propene
C. But-2-ene
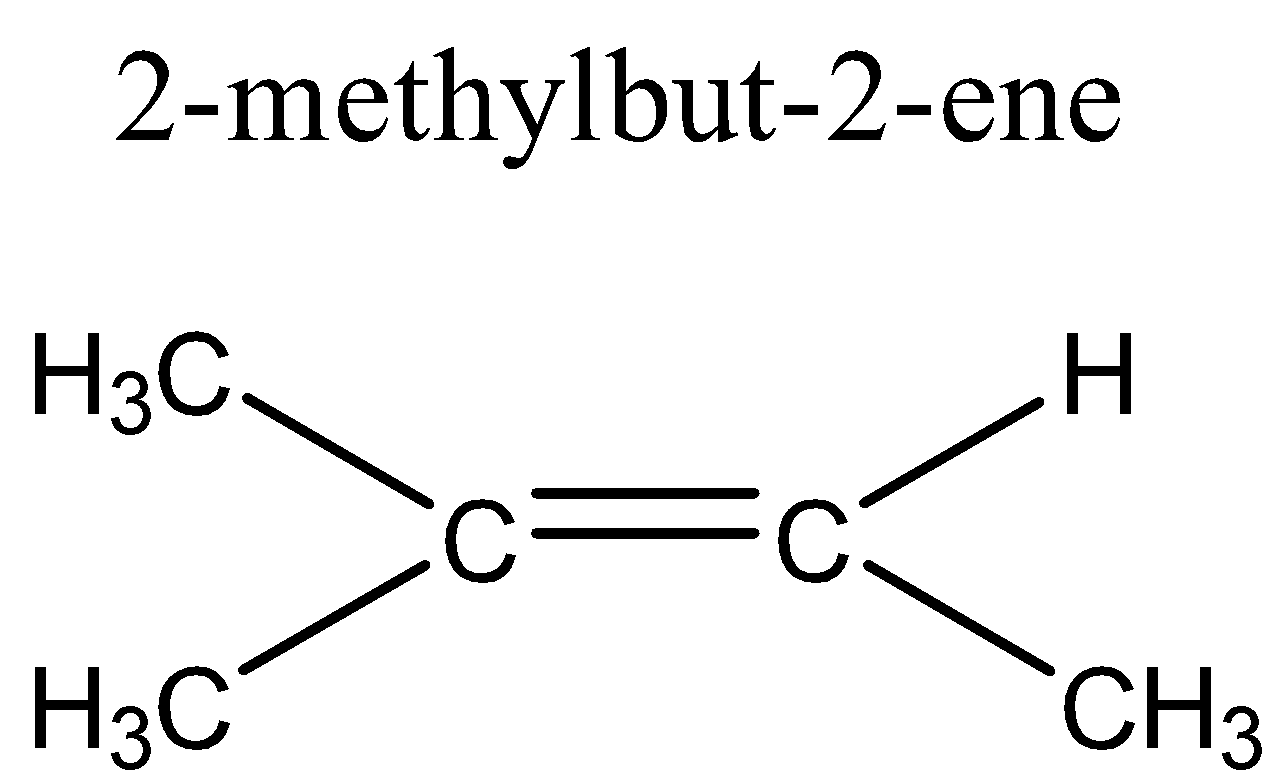
D. 2-methylbut-2-ene
Solution
We know that geometrical isomerism is shown by the compounds having the same molecular formula but differ in the arrangement of atoms in space (around double bonds). It is also known as Cis and Trans isomerism or configurational isomerism.
Complete step by step answer:
As we have studied, isomers are molecules with the same molecular formula. Isomerism is the spatial arrangement of atoms having the same molecular formula but differ in physical and chemical properties.
There are two types of isomerism, one is structural isomerism and other is stereoisomerism.
In structural isomerism the isomers have the same molecular formula but their structure is different.
E.g., Chain isomerism, position Isomerism.
In stereoisomerism, the molecules have the same molecular but differ in spatial arrangement. E.g., Geometrical isomerism and optical isomerism.
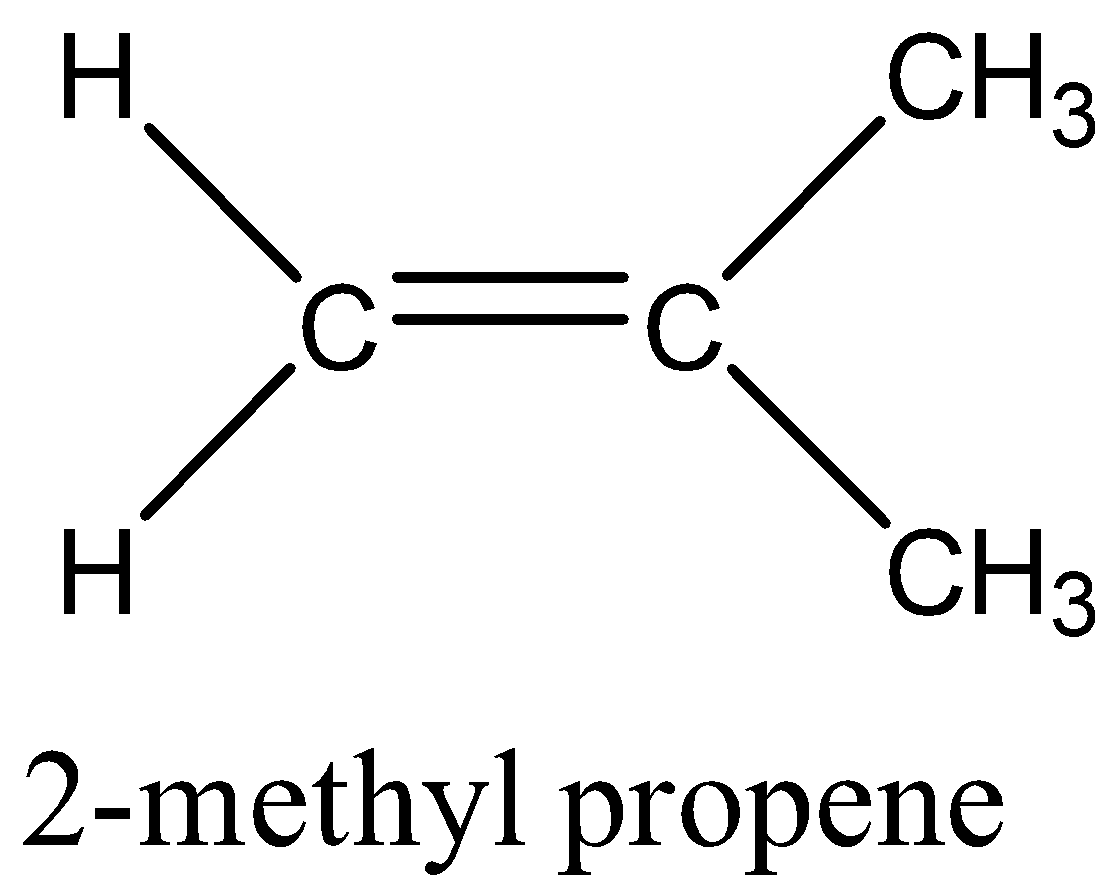
In option A, 2- methylpropene, the groups attached to double bonded carbon atoms are same that is H,H on one carbon and CH3,CH3 on other carbon, so if we rotate them still the groups will remain same, thus this alkene cannot show geometrical isomerism.
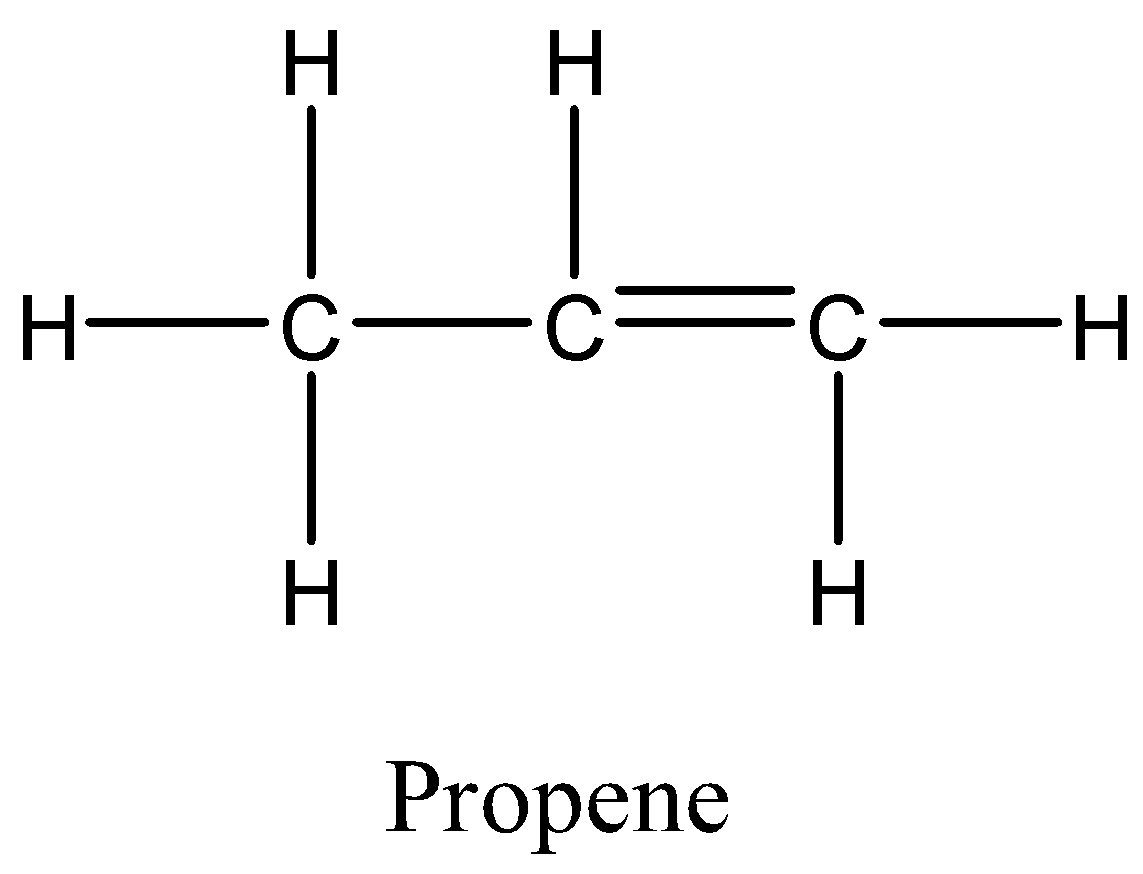
In option B, Propene also has hydrogens attached to both the carbon atoms that means all the groups are the same again. So, this alkene will not exhibit geometrical isomerism.
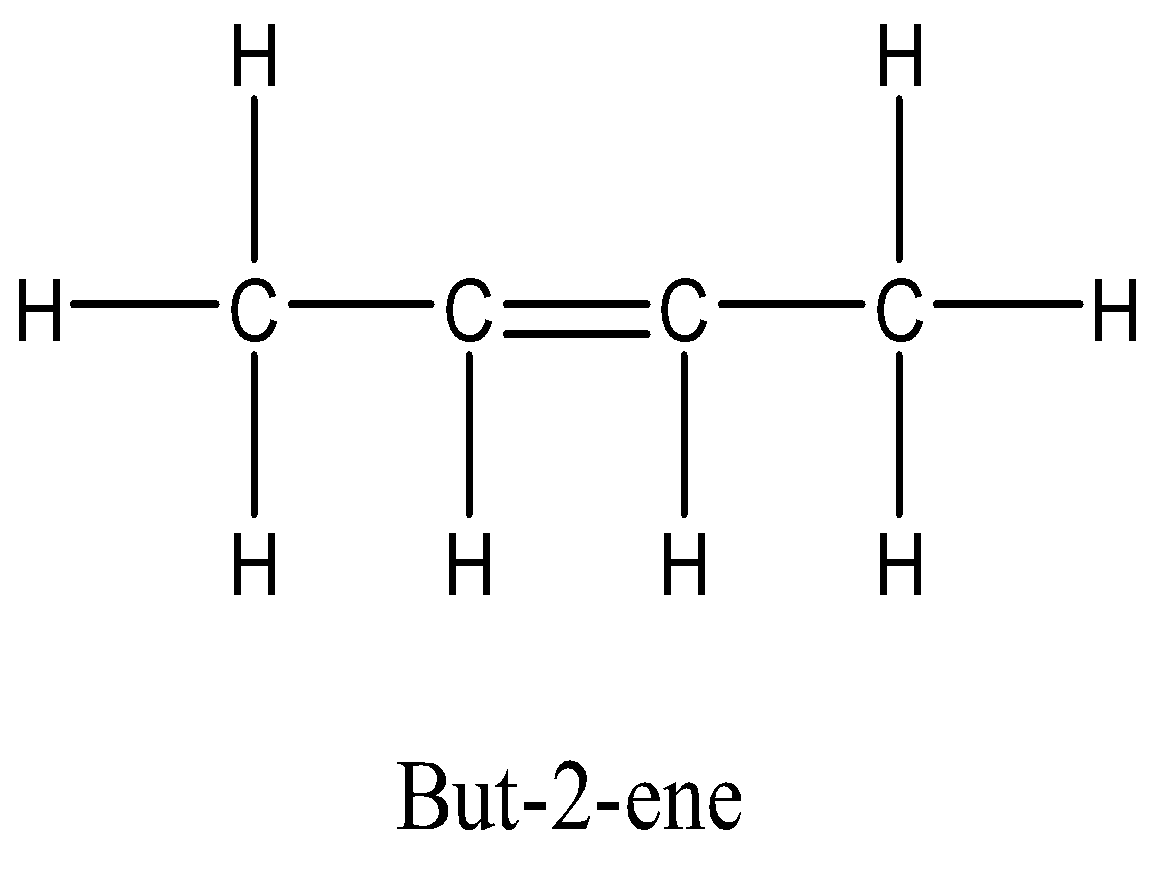
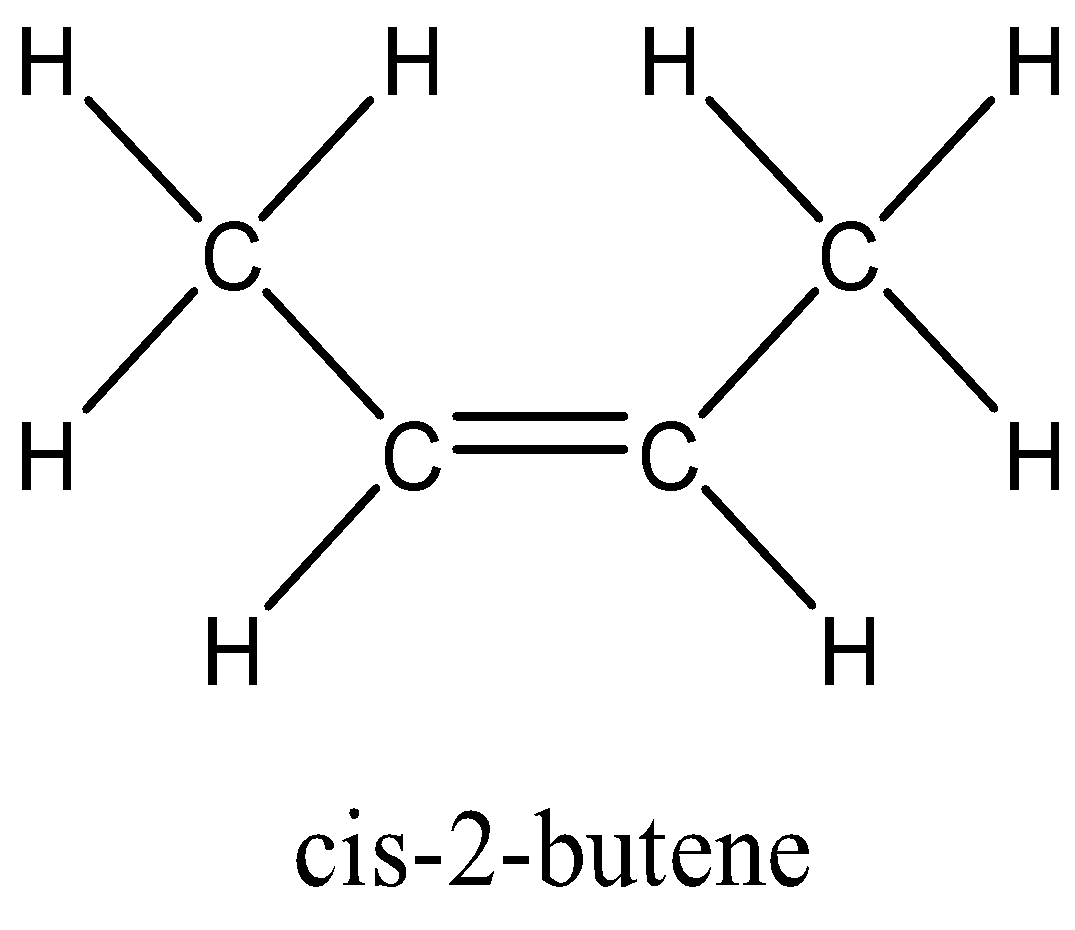
In option C, that is But-2- ene, the groups attached to double bonded carbon are different, that is H and CH3 on one carbon and other side also has H and CH3 can be rotated on same side of double bond to form cis-2- butene and on rotating opposite sides of double bonded carbon atom it forms trans-2- butene. So, the alkene But-2-ene shows geometrical isomerism.
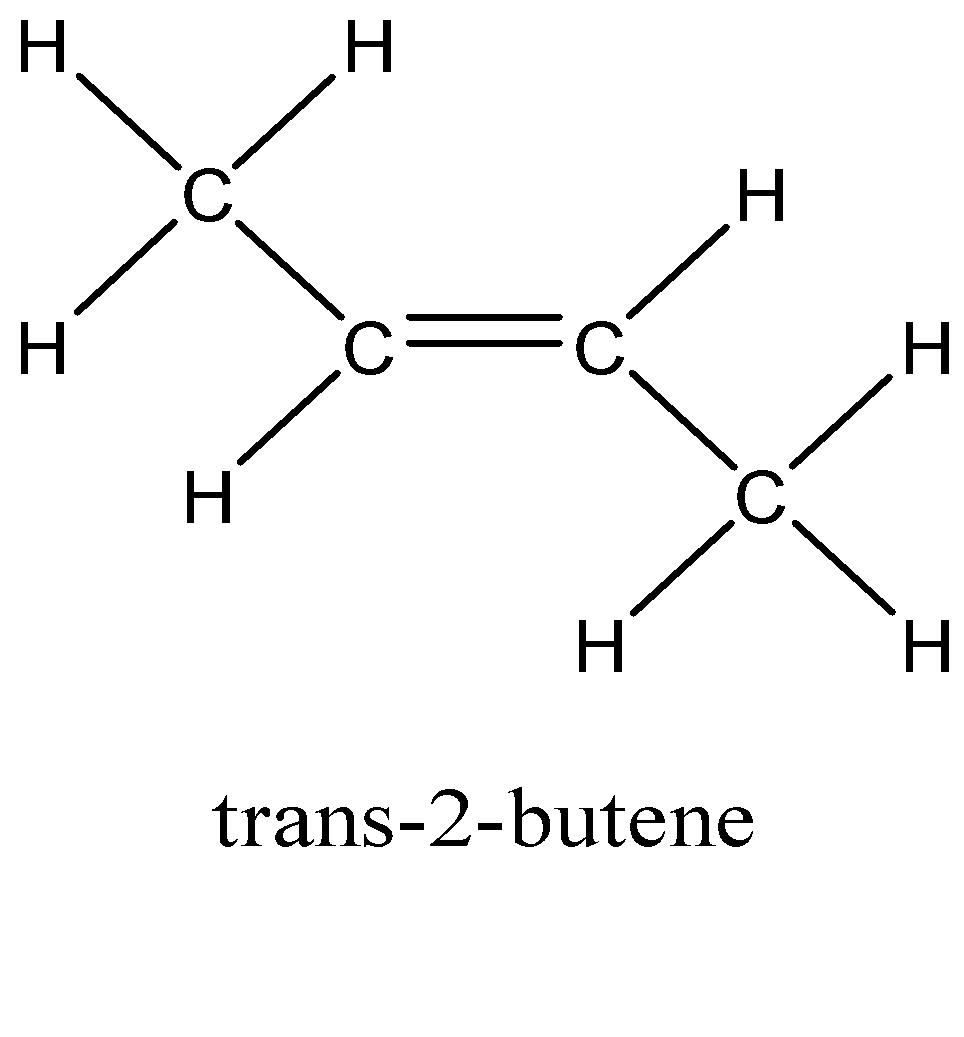
In option D, 2-methylbut-2-ene, there are two methyl groups attached to one of the double bonded carbon atoms, if rotated cannot show geometrical isomerism.
So, the correct answer is Option C.
Note: We must remember that in order to identify geometrical isomers in any compound one should look at the groups attached near the double bond. The rotation around double or single bonds is restricted. These isomers are also denoted by E-Z also, that is if groups are on the same side it is denoted as Z- isomer and if groups are on opposite sides it is known as E isomer.
