Question
Question: The alkene ligand ($\pi$-C$_2$R$_4$) is both a '$\sigma$' donor and a '$\pi$' acceptor, similar to t...
The alkene ligand (π-C2R4) is both a 'σ' donor and a 'π' acceptor, similar to the CO ligand in metal carbonyls, and exhibits synergic bonding with metals. Correct order of C-C bond length in K[PtCl3(π-C2R4)] complexes in which R = H, F or CN is
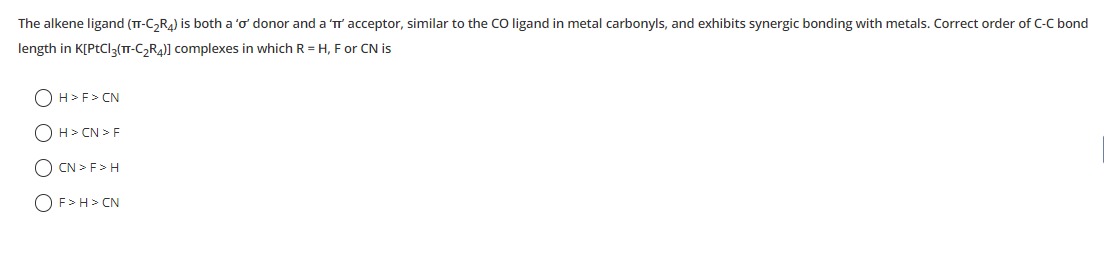
H > F > CN
H > CN > F
CN > F > H
F > H > CN
CN > F > H
Solution
The bonding in metal-alkene complexes like K[PtCl3(π-C2R4)] involves synergic bonding, similar to metal carbonyls. This involves two main components:
- σ-donation: The alkene donates electron density from its filled π orbital to an empty d orbital of the metal.
- π-back-donation: The metal donates electron density from its filled d orbital to the empty π∗ (antibonding) orbital of the alkene.
The π-back-donation is crucial for the C-C bond length in the alkene. When electron density is donated into the π∗ antibonding orbital of the C=C bond, it weakens the C-C π bond and consequently increases the C-C bond length.
The extent of π-back-donation depends on the electron-accepting ability of the alkene. An alkene with more electron-withdrawing substituents (R) will have a lower energy (more stable) π∗ orbital, making it a better acceptor for electron density from the metal.
Let's compare the electron-withdrawing nature of the substituents R:
- R = H (Hydrogen): Hydrogen is neither strongly electron-donating nor electron-withdrawing.
- R = F (Fluorine): Fluorine is a highly electronegative atom and exerts a strong inductive electron-withdrawing effect (-I effect).
- R = CN (Cyano group): The cyano group is a strong electron-withdrawing group due to both its inductive effect (-I) and its resonance/mesomeric effect (-M), as it has an empty π∗ orbital that can accept electron density. In general, the -CN group is considered a stronger electron-withdrawing group than -F. For instance, comparing Hammett σp values: σp(H)=0, σp(F)=+0.06, σp(CN)=+0.66.
Based on the electron-withdrawing strength, the order is:
CN > F > H
A stronger electron-withdrawing R group makes the alkene a better π-acceptor.
Better π-acceptor alkene ⇒ Greater extent of π-back-donation from metal to alkene π∗.
Greater π-back-donation ⇒ More electron density in the C-C antibonding orbital.
More electron density in antibonding orbital ⇒ Weaker C-C bond ⇒ Longer C-C bond length.
Therefore, the order of C-C bond length will be directly proportional to the electron-withdrawing strength of R:
C-C bond length order: C2(CN)4 > C2F4 > C2H4
This corresponds to the order: CN > F > H.
