Question
Question: The aldehyde which will not form Grignard product with one equivalent Grignard reagent are- * ...
The aldehyde which will not form Grignard product with one equivalent Grignard reagent are-
-
A.
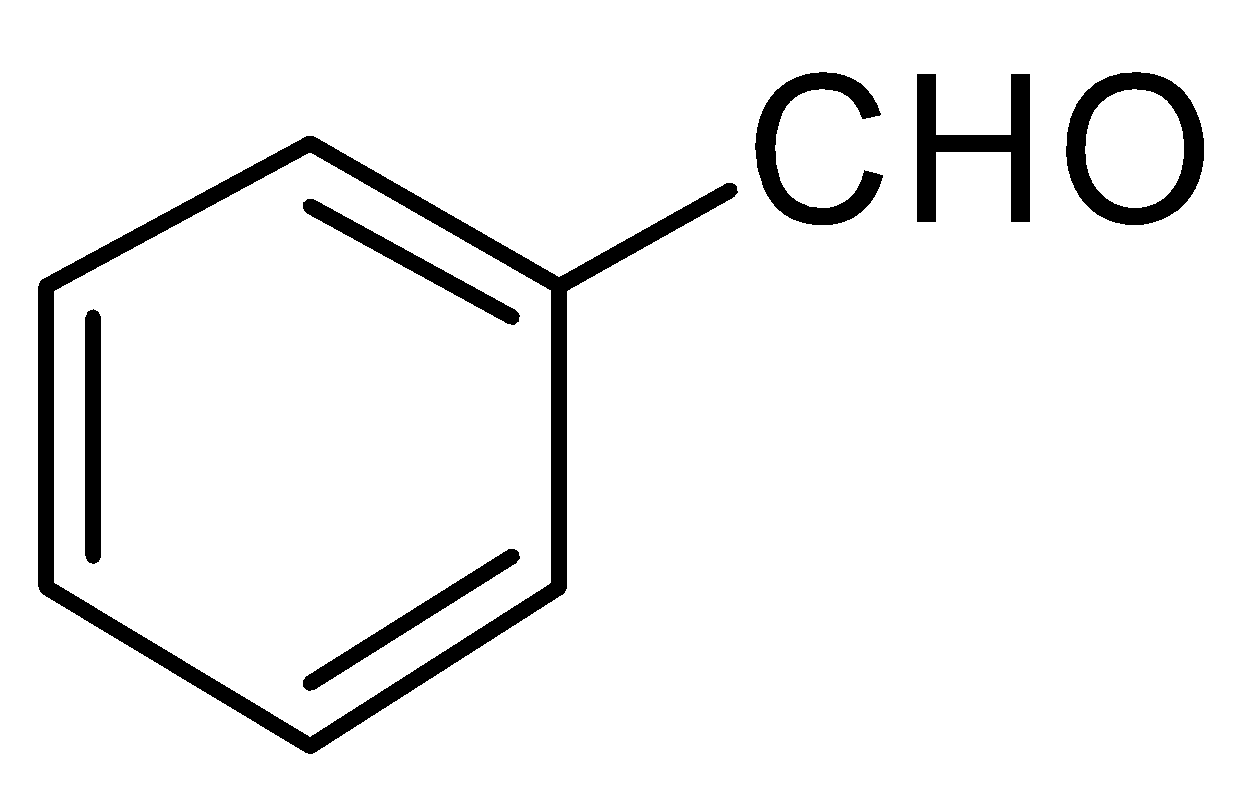
-
B.
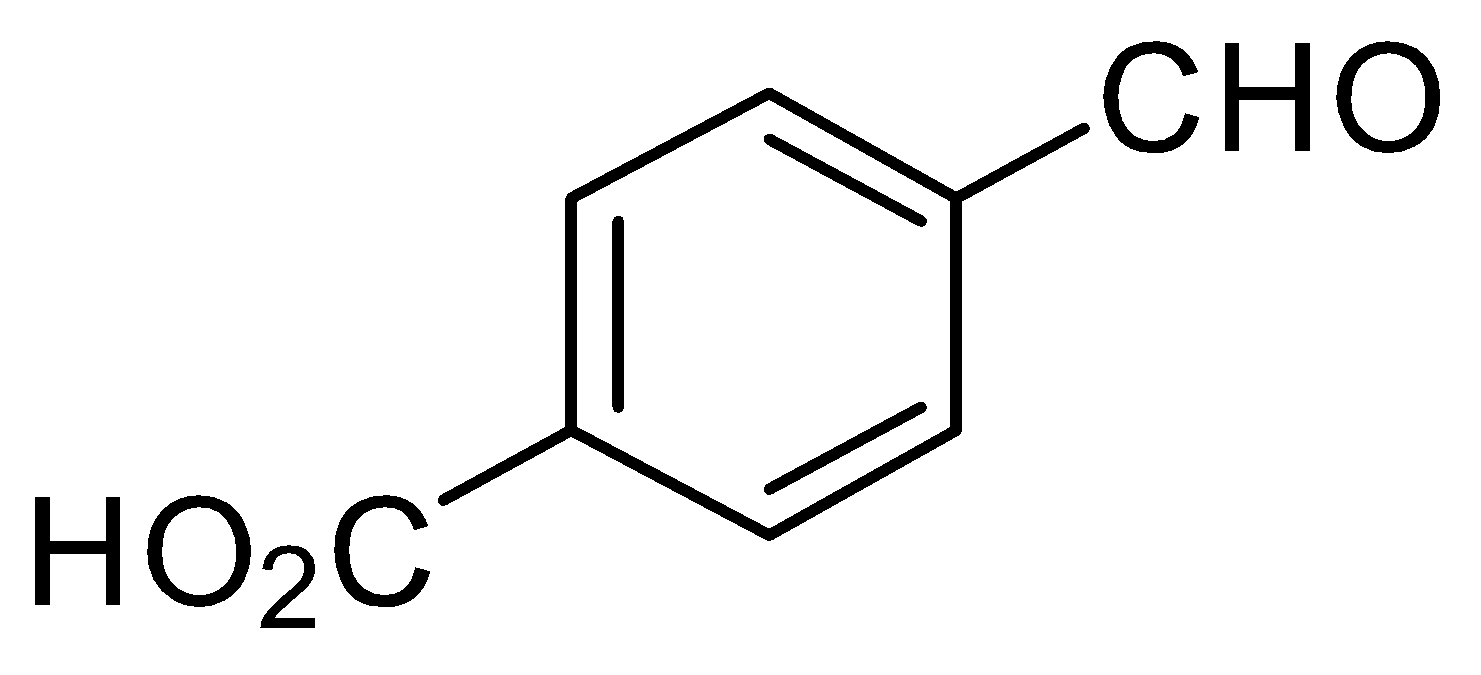
-
C.
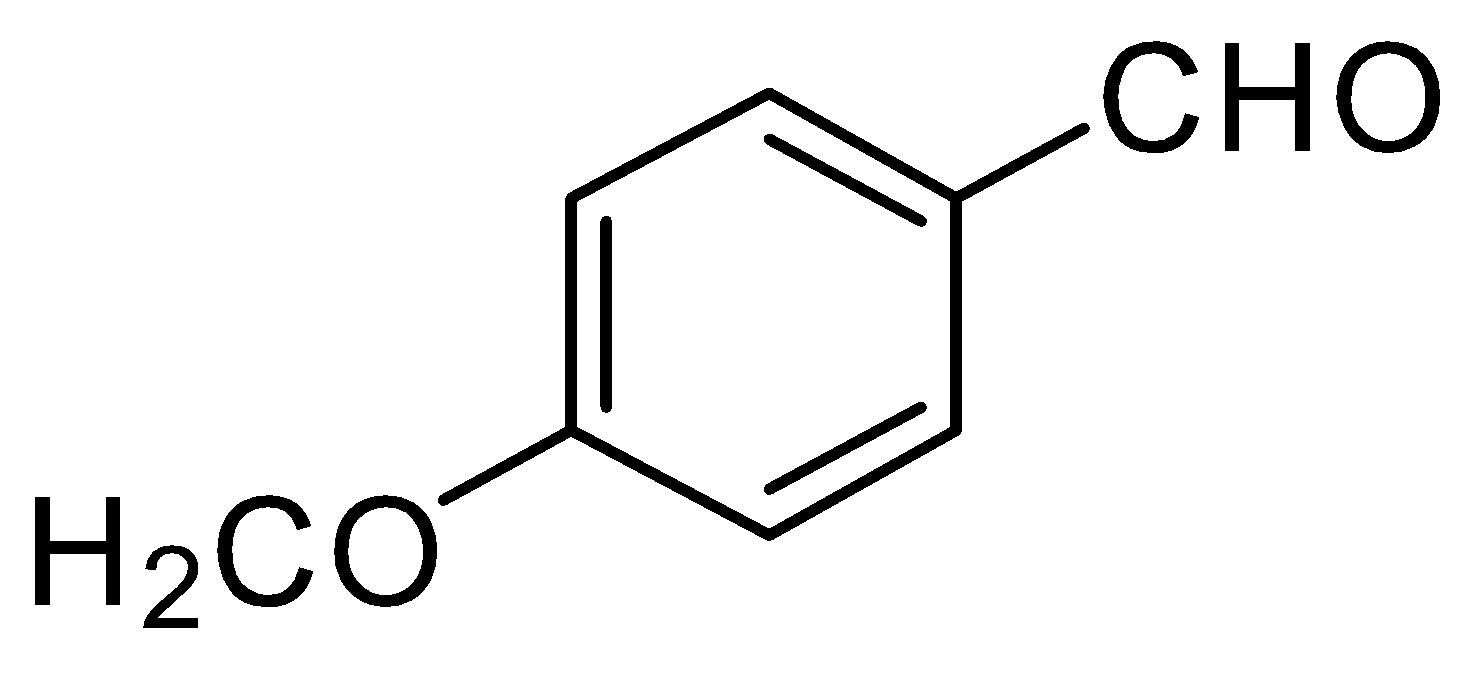
-
D.
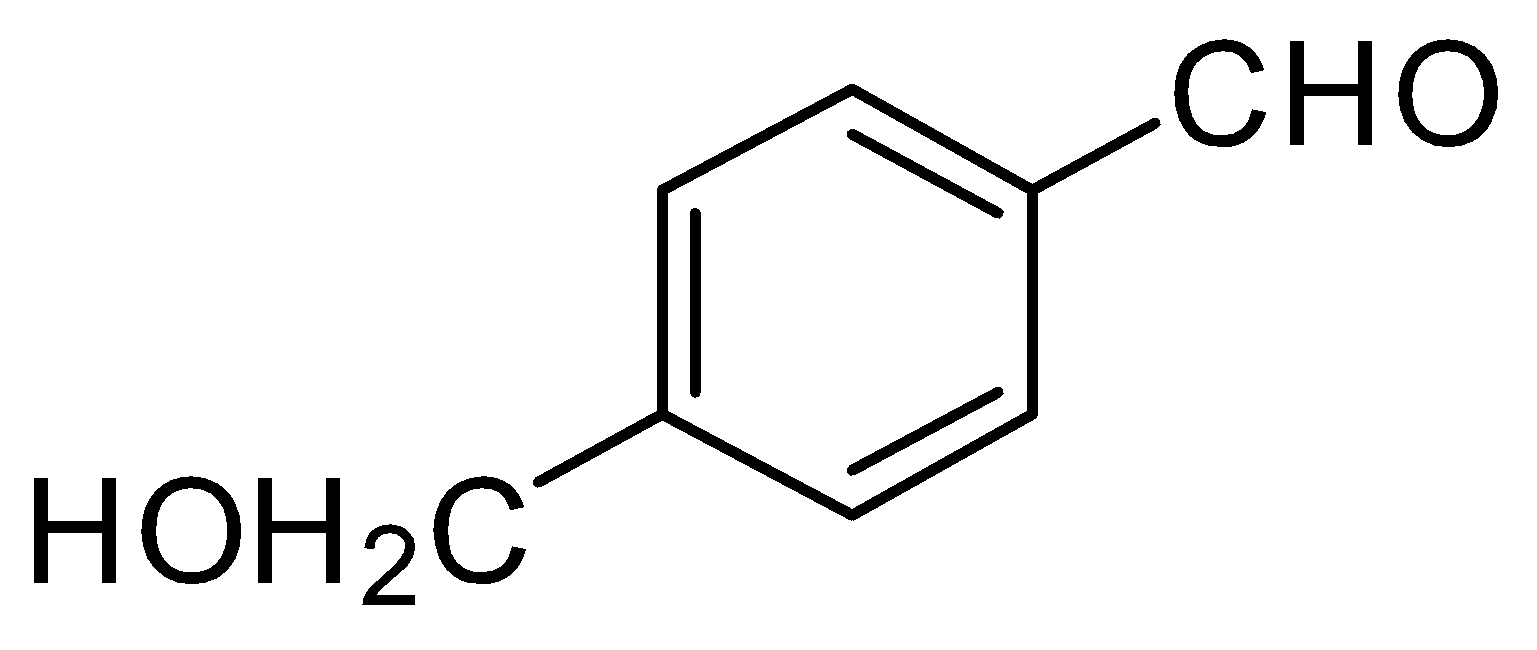
(1) (B),(C),(D)
(2) (B),(D)
(3) (B),(C)
(4) (C),(D)
Solution
Hint : Grignard reaction mechanism refers to the reaction between an aldehyde or ketone with Grignard reagent. Basically, Grignard reaction is an addition reaction in which addition of Grignard reagent takes place to an aldehyde or ketone.
Complete Step By Step Answer:
A Grignard reagent is an organic reagent which was discovered by scientist Victor Grignard. It is an organo-magensium compound which has the chemical formula of ′R−Mg−X′ in which R refers to an alkyl group or aryl group and X refers to a halogen. This reagent involves a magnesium metal and alongside of magnesium metal, there is a halogen atom and an alkyl or aryl group is attached. The Grignard reactions are prepared when an alkyl or aryl halide reacts with magnesium and it undergoes a transformation in which the electrophilic alkyl halide converts into nucleophilic carbanion molecules. The diethyl ether is an important ether solvent for the synthesis of Grignard reagent. The reactivity of halogens decreases in the series of I>Br>Cl>F . The fluorine has least reactivity so organic fluorides are not used for the synthesized Grignard reagent.
It is the most important reagent in organic chemistry. It is very useful in forming carbon-carbon bonds. When the Grignard reagent reacts with aldehyde or ketone, it forms alcohol.
An example of reaction of Grignard reagent with aldehyde:
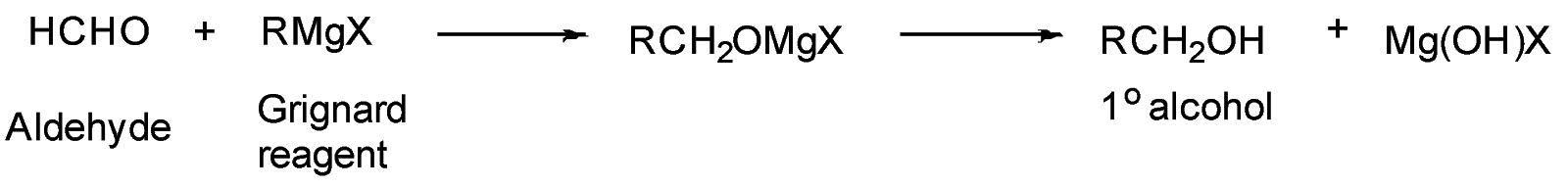
Grignard reagent (R−Mg+−X−) also act as a base and abstract acidic proton (H+) to give (R−H) .
In the given question, only in (B) and (D) compounds have an acidic proton (H+) . Due to which Grignard reagent gets destroyed. So, they will not form the Grignard product with one equivalent of Grignard reagent. Here are the reaction below:-
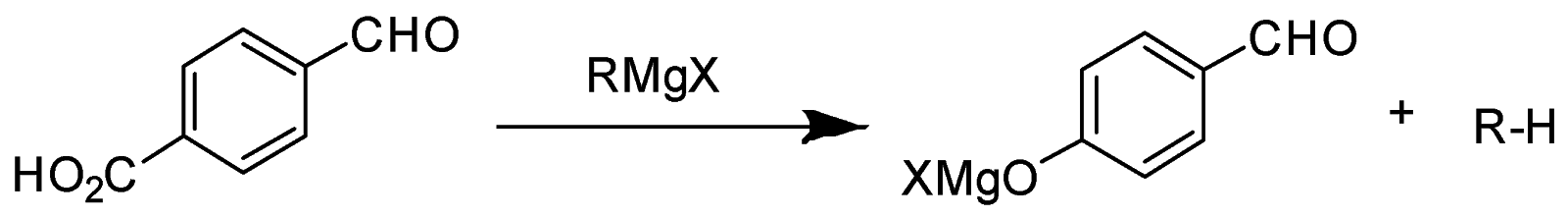
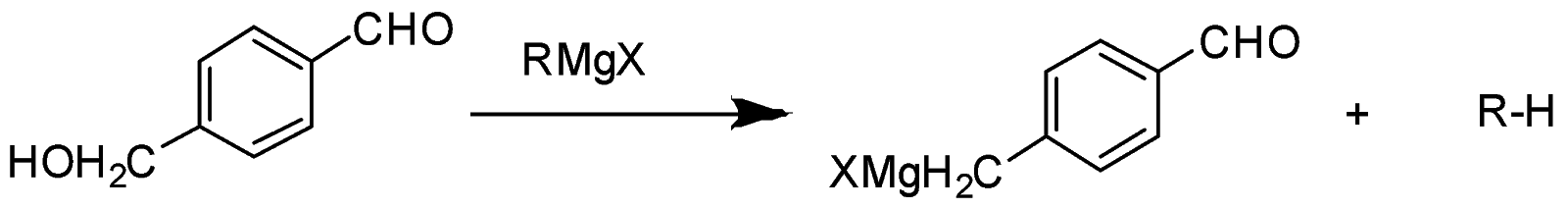
Therefore, the other two will give a nucleophilic addition reaction with Grignard reagent.
Hence, the correct answer is option (2) .
Note :
It should be remembered that Grignard reagents cannot react with water and alcohol. This is because water or alcohol would protonate and thus destroy the Grignard reagent. Grignard reagent acts as a strong nucleophile and forms a hydrocarbon.
