Question
Question: The alcohol which gives immediate turbidity with Lucas reagent is: (A) 
(B)
(C)
(D)
Solution
Hint:Lucas reagent is a solution of concentrated HCl containing anhydrous Zinc Chloride. This reagent is used in the Lucas test, which is used to distinguish between primary, secondary, and tertiary alcohols.
Complete step-by-step answer:
-Lucas test is a test that is used to distinguish between alcohols. It is actually the difference in the reactivity of the three alcohols with HCl that distinguishes them from one another.
- In this reaction, alcohols react with hydrogen halides to form alkyl halides. The general equation is written as follows:
ROH+HX→RX+H2O
- In case of tertiary alcohols, tertiary carbocations are involved, in case of secondary alcohols, secondary carbocation is involved and in case of primary alcohols, primary are involved.
- We know that tertiary carbocations are more stable than secondary which in turn are more stable than primary carbocations. So greater the stability of carbocation, greater will be its ease of formation from an alkyl halide and faster will be the rate of reaction.
- As primary carbocations are the least stable, hence at room temperature they do not give Lucas test.
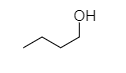
Butanol is a primary alcohol, so it will not show turbidity immediately.
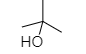
- 2-methyl propan-2-ol is tertiary alcohol and as we know that tertiary alcohols show turbidity immediately and they give Lucas test.
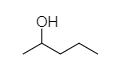
- Pentan-2-ol is a secondary alcohol, so it will show turbidity after some minutes.
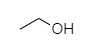
- Ethanol is a primary alcohol, so it will not show turbidity immediately.
As we know that tertiary alcohols give turbidity immediately, so the correct option is B.
Note: The possibility to make a mistake is that you may choose option C. As secondary alcohol also gives Lucas test but in this, the turbidity appears after some minutes not immediately.
