Question
Question: The alcohol that produces turbidity immediately with Lucas reagent at room temperature. A. \[1 - h...
The alcohol that produces turbidity immediately with Lucas reagent at room temperature.
A. 1−hydroxy butane
B. 2−hydroxy butane
C. 2−hydroxy−2−methyl propane
D. 1−hydroxy−2−methyl propane
Solution
Alcohols react with Lucas reagent (ZnCl2/Conc. HCl)in the nucleophilic substitution mechanism.
Different types of alcohol react differently with Lucas reagent.
So, this problem is basically based on the concept of reactivity of different types of alcohols with Lucas reagent.
Complete step by step answer:
As we all know, Lucas reagent is used to differentiate between Primary (10) Secondary (20) and Tertiary(30) alcohols.
Now we will see Lucas reagent reacts differently with different types of alcohols-
10 Alcohols ZnCl2Conc.HClPrecipitate forms after 10 minutes
20 Alcohols ZnCl2Conc.HCl Precipitate forms within 5−10 minutes
30 Alcohols ZnCl2Conc.HCl Precipitate forms immediately
Now we will see the mechanism of the reaction-
If the nucleophilic substitution gets completed in two steps, i.e.$$$$S_N^{{\text{ 1}}} mechanism-
R - OH R+ X−R - X (Racemic mixture)
If the nucleophilic substitution gets completed in one step, i.e. SN 2 mechanism-
X− + R - OH [X - - - R - - - OH] X - R
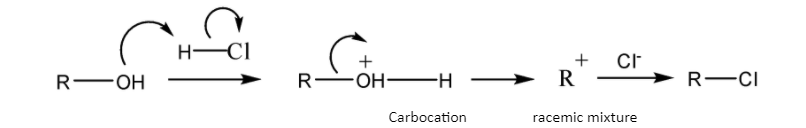
Schematic representation of SN 1 mechanism-
Schematic representation of SN 2 mechanism-
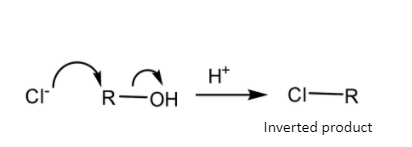
So, theSN 1 mechanism gives a carbocation during the course of reaction but the SN 2 mechanism gets completed in one step so it does not give any such carbocation during the course of reaction.
The reactivity of alcohols in the SN 1 mechanism -30- alcohol > 20- alcohol >10- alcohol
The reactivity of alcohols in the SN 2 mechanism - 10- alcohol> 20- alcohol>30- alcohol
So, Lucas reagent reacts with alcohols in SN 1 mechanism, where the 30-alcohol reacts immediately.
Now, the structures of the given options-
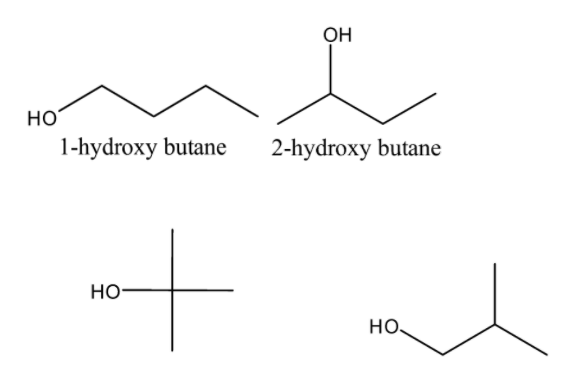
From the above structures, it is clear that Option-C i.e.2−hydroxy−2−methylpropane is a tertiary alcohol.
So option-C is the correct answer.
Note: Remember, SN 1 mechanism gives a carbocation in the1ststep, which subsequently reacts with the nucleophile resulting the racemic mixture. Because a carbocation has one vacant p-orbital which can accept the nucleophile using both the lobes of the p-orbital. So, the rate of reaction depends on the stability of the carbocation.
On the other hand, in SN 2mechanism, no such carbocation forms. So, the rate of reaction depends on the availability of the Carbon atom of the substrate molecule.
