Question
Question: The action of sodium metal on a mixture of aryl halide and alkyl halide in ether gives arenes. A. ...
The action of sodium metal on a mixture of aryl halide and alkyl halide in ether gives arenes.
A. True
B. False
Solution
The aromatic hydrocarbons are called as arenes. A reaction in which aryl halide and alkyl halide react together in presence of sodium metal and dry ether to form alkyl benzene is known as the Wurtz-Fittig reaction. In this reaction, aryl halides undergo alkylation. As new C−C bond forms in this reaction, this is known as a coupling reaction.
Complete answer:
-When sodium metal reacts with the mixture of aryl halide and alkyl halide in presence of ether, arenes form as the product. The reaction is known as the Wurtz-Fittig reaction. For example, when bromobenzene reacts with methyl bromide in presence of sodium and dry ether, it forms ethylbenzene which is also known as toluene. The reaction can be written as below
C6H5−Br+CH3Br2Na/dryetherC6H5−CH3+2NaBr
An aromatic hydrocarbon that has an alternative single and double bond between the carbon atoms is known as arene. From the structure of methylbenzene or toluene, we can see, it has the C6H5- ring which contains alternative double bonds with a methyl group attached to it, and hence it is an arene.
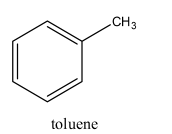
Thus, the correct option is, (A).
Note:
- This reaction has the best use to form an asymmetric product. Generally, it is used for the alkylation of aryl halides.
-With the use of ultrasound in this reaction, biphenyl compounds can also be formed.
-This reaction is useful for synthesizing organosilicon compounds in the laboratory.
-Another important thing to be noted is instead of sodium metal, other metals can be used in this reaction such as Cu, K, and Fe.
-However, this reaction has very limited applicability as side reactions such as elimination and rearrangement reactions are prevailing.
