Question
Question: The acid which contains both -OH and -COOH groups is: A. Phthalic acid B. Adipic acid C. Gluta...
The acid which contains both -OH and -COOH groups is:
A. Phthalic acid
B. Adipic acid
C. Glutaric acid
D. Salicylic acid
Solution
This question can only be attempted after the structure of these acids are drawn completely so that we can easily know which of these acids contain both the alcohol and carboxylic acid groups.
Complete Solution :
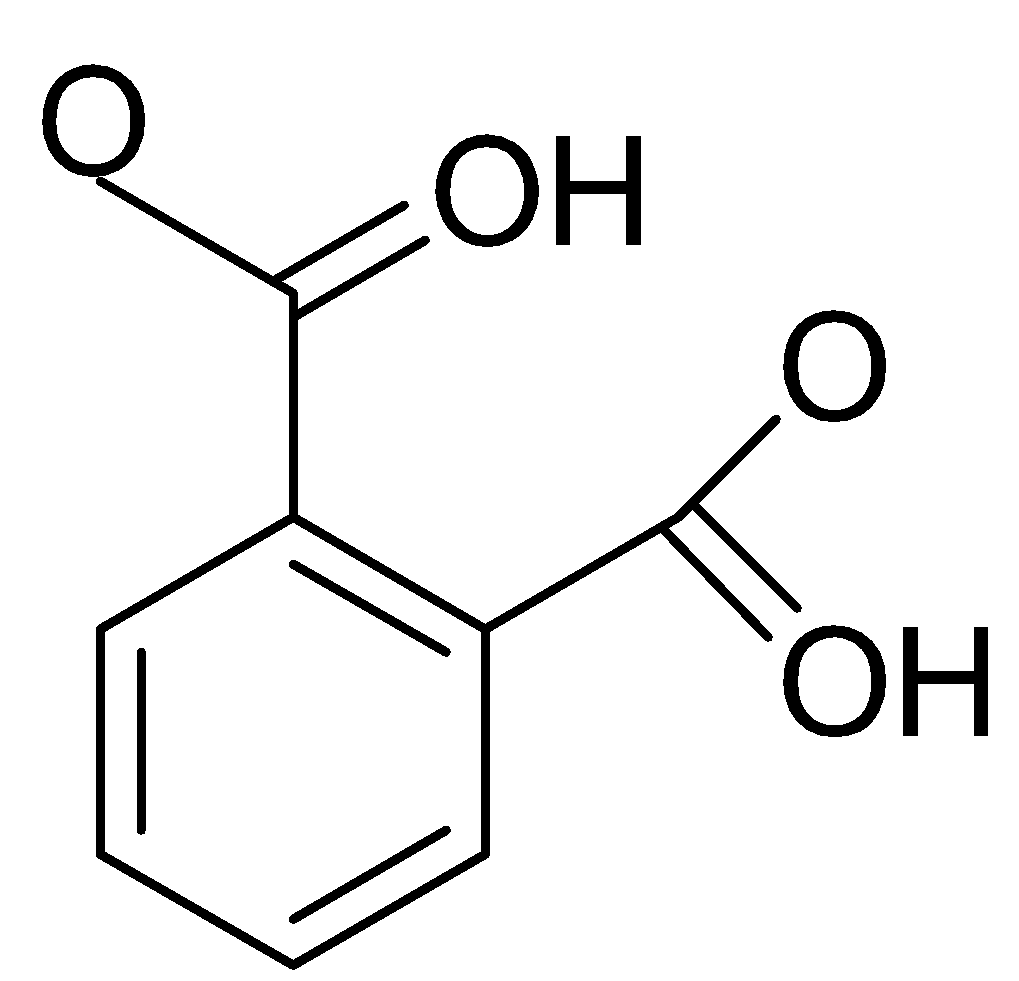
If we look at first acid which is phthalic acid which is also known as Benzene-1,2-dicarboxylic acid with its formula C6H4(CO2H)2. It is also known as the isomer of isophthalic and terephthalic acid. When we look at the structure of this acid, we will notice that there are only COOH groups present.
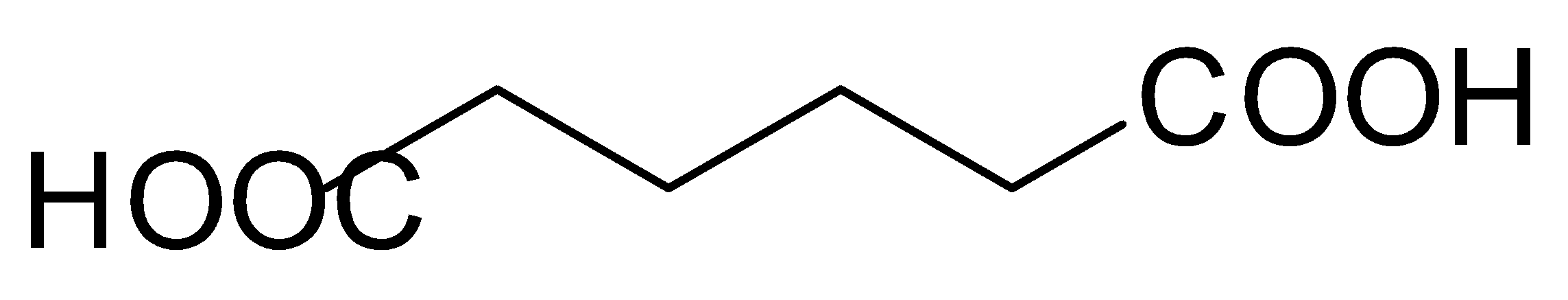
Moving on to the adipic acid, it is also known as dibasic acid or the palindrome acid, because both the sides of this acid are very similar and mostly it is used in the manufacturing of nylons. If we look at the structure of this acid, then it also contains carboxylic acid on both the ends and no alcohol group.
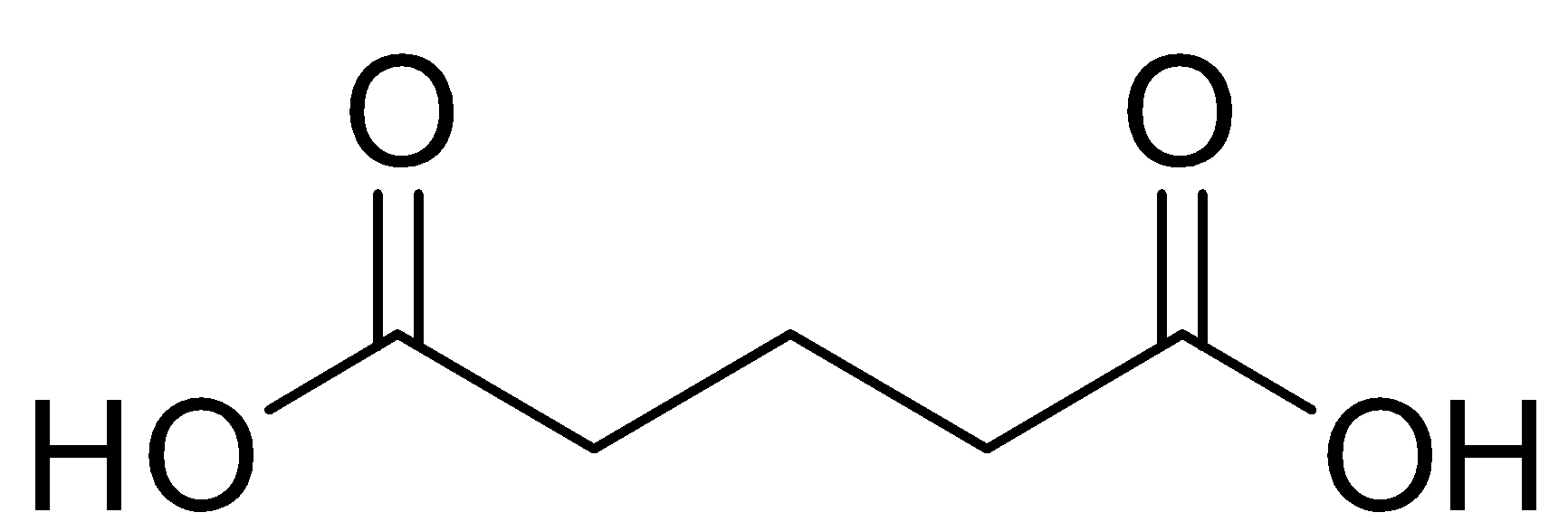
Next acid is glutaric acid , it is also known as linear dibasic acid and it is also naturally produced in the body in the midst of a cycle, and is water soluble, if we look at the structure of glutaric acid, we will notice, there is carboxylic acid group present again on both the sides
Only the salicylic acid is left, it has very wide beneficial medicinal effects such as it is the major component in aspirin and it is widely used in treating acne or pimples. It’s also known as monohydroxy benzoic acid and a type of phenolic acid. If we look at the structure then, we will notice that it contains both the -OH and -COOH groups.
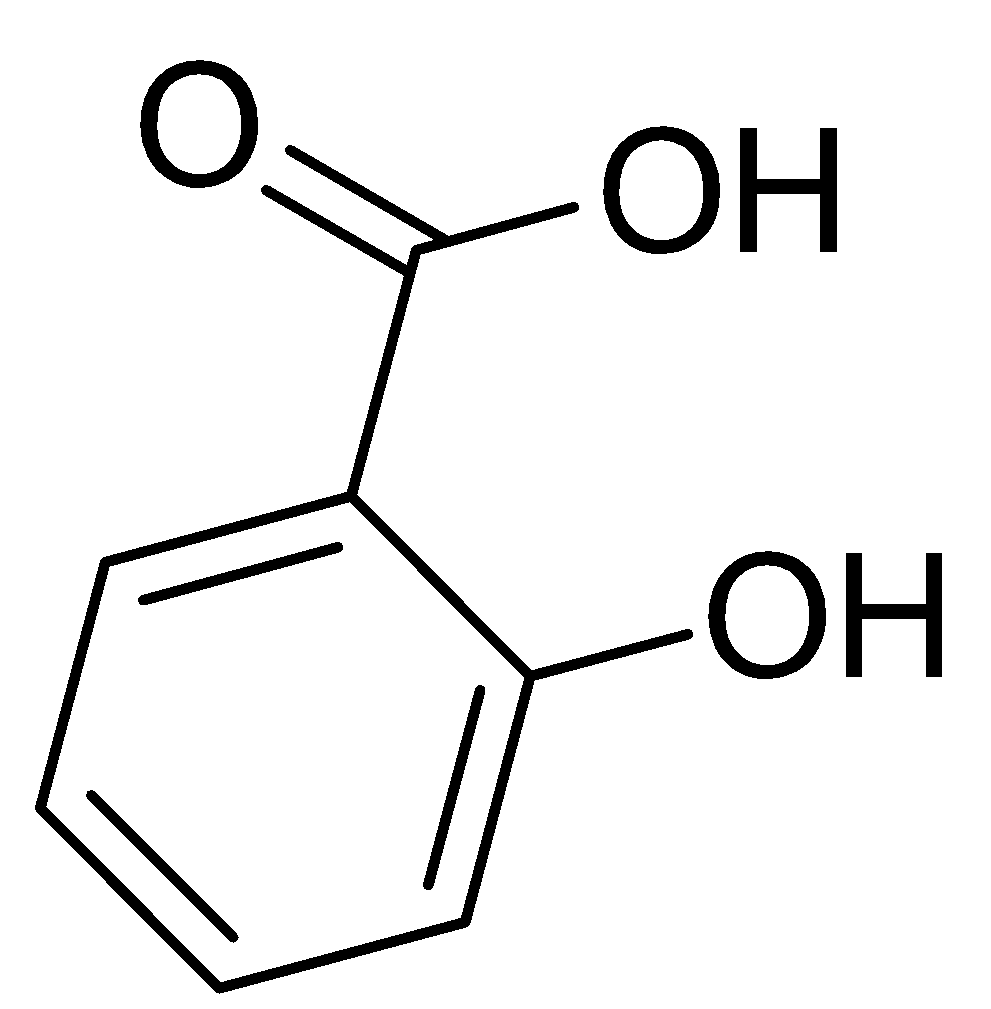
Therefore, the answer of this question is very clear, the acid which contains both the -OH and -COOH
So, the correct answer is “Option D”.
Note: First three acids belong to the carboxylic acid family itself as they are formed from either the isomers or polymers of acids which mainly contain carboxylic acids on both the sides however, salicylic acid belongs to the phenolic part.
