Question
Question: The acid used in the making of vinegar is: A.Formic acid B.Acetic acid C.Sulphuric acid D.Ni...
The acid used in the making of vinegar is:
A.Formic acid
B.Acetic acid
C.Sulphuric acid
D.Nitric acid
Solution
Vinegar is obtained by a process known as fermentation. For producing vinegar, ethyl alcohol or ethanol is fermented by bacteria from the family known as Acetobacteraceae.
Complete step by step answer:
Vinegar can be very well defined as a dilute solution of ethanoic acid in water. Ethanoic acid is also more commonly known as acetic acid.
Vinegar is a naturally occurring compound which is in the liquid state, so there is no defined concentration for it. Although we have a range of about 5% to 20% concentration of acetic acid in water. The chemical formula for vinegar thus obtained is CH3COOH. So vinegar is basically a diluted solution of acetic acid in water. Because of this, vinegar is considered to be a weak acid. The molecular structure of vinegar can be given as:
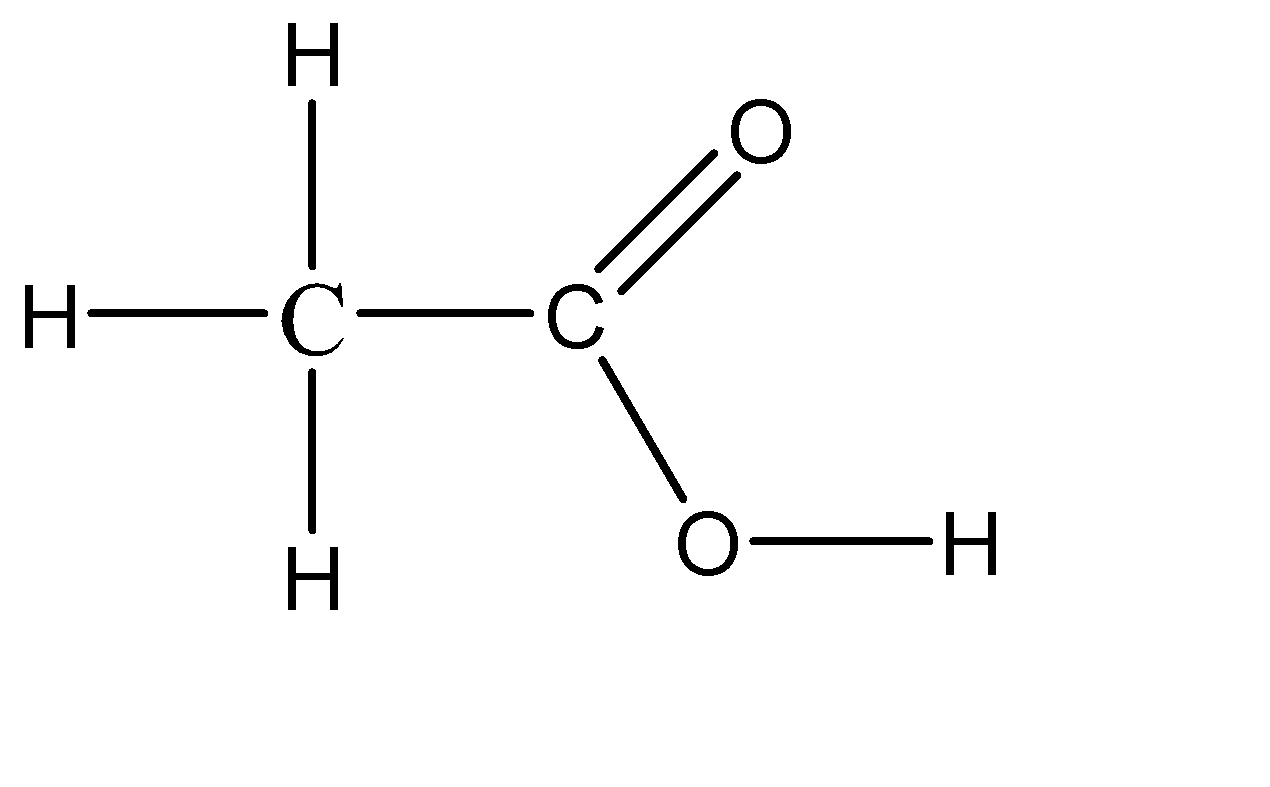
Hence, the acid used in the making of vinegar is acetic acid.
Hence, Option B is the correct option.
Note:
Although the acidity of this compound is low, i.e. it has a very low pH value, even then the acetic acid does not show complete dissociation in water.
